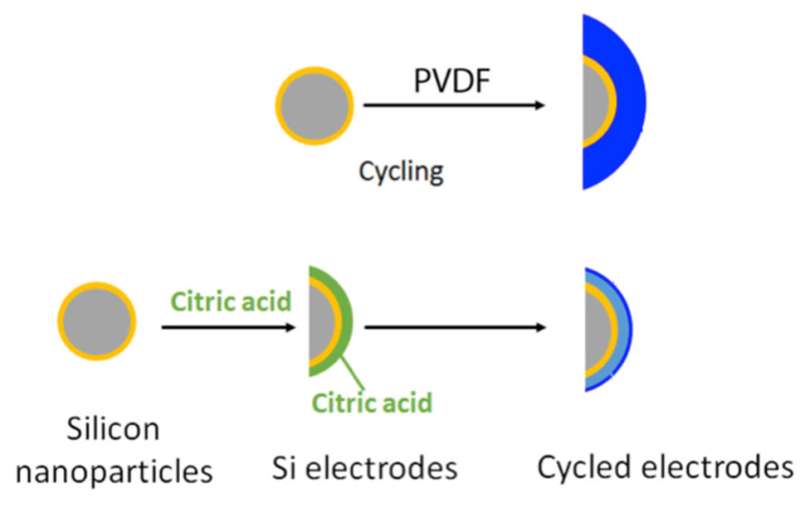
Battery electrodes composed of silicon (Si) nanoparticles (gray with a shell of surface oxide in yellow) were more resilient to charge/discharge cycling with the addition of surface-binding citric acid (green) than with poly(vinylidene) fluoride (PVDF), a widely used binder in other lithium-ion batteries. During cycling (right), the citric acid reacted to form a protective layer (light blue), which suppressed the decomposition of the electrolyte. The resulting solid electrolyte interphase (dark blue) was thinner and more stable. Credit: Brett Lucht, University of Rhode Island
In your electric car's battery, swapping an electrode with one made of silicon could let the battery store 10 times more energy. Why isn't silicon used? It falls apart. Scientists designed binders, small molecules and polymers, to modify the surface chemistry of the silicon. The binders improved resilience to cycling. A binder-based layer was formed during electrode preparation and initial cycling. The binder layer protected the surface.
The research is developing a better understanding of modified silicon electrodes for lithium-ion batteries. The result? The work could lead to batteries that give electric vehicles a greater driving range.
Lithium-ion batteries with silicon anodes have a theoretical capacity 10 times higher than the most commonly used anodes. However, silicon electrodes suffer degradation during charging and discharging. The electrode contracts and expands during cycling, pulverizing active particles and destroying electrical contact. The volume changes break down and destroy the solid electrolyte interphase. A team led by researchers from the University of Rhode Island investigated reactive carboxylic acid functionalized polymers and small molecules as surface-modifying agents and binders for silicon anodes. Small molecules have the added benefit of high orientational flexibility for better surface coverage. All the functional binders modified the surface of the silicon nanoparticles.
During cycling, these binders electrochemically reacted and formed a protective layer, which suppressed the decomposition reactions with the electrolyte. The resulting solid electrolyte interphase was thinner than with the widely used binder poly(vinylidene) fluoride. Remarkably, after only one charge/discharge cycle, the small molecule, citric acid, reacted and formed a layer that protected the silicon from reacting with the electrolyte. Along with other binders, citric acid allowed for improved capacity retention and a more stable solid electrolyte interphase. The surface-modified silicon has improved cycle life, making these materials more attractive for next-generation lithium-ion batteries and long-range electric vehicles.
More information: Cao Cuong Nguyen et al. Systematic Investigation of Binders for Silicon Anodes: Interactions of Binder with Silicon Particles and Electrolytes and Effects of Binders on Solid Electrolyte Interphase Formation, ACS Applied Materials & Interfaces (2016). DOI: 10.1021/acsami.6b03357
Cao Cuong Nguyen et al. Improved Cycling Performance of a Si Nanoparticle Anode Utilizing Citric Acid as a Surface-Modifying Agent, Langmuir (2016). DOI: 10.1021/acs.langmuir.6b04310
Journal information: Langmuir , ACS Applied Materials and Interfaces
Provided by US Department of Energy