Battery breakthrough using 2016 Nobel Prize molecule
Silicon anodes are receiving a great deal of attention from the battery community. They can deliver around three to five times higher capacity compared with those using current graphite anodes in lithium ion batteries. A higher capacity means longer battery use per charge, which is particularly critical in extending the driving mileage of all-electric vehicles. Although silicon is abundant and cheap, Si anodes have a limited charge-discharge cycle number, which is typically less than 100 times with microparticle sizes. Their volume expands enormously during each charge-discharge cycle, leading to fractures of the electrode particles or delamination of the electrode film equally, even in decaying its capacity.
A KAIST research team led by Professors Jang Wook Choi and Ali Coskun reported a molecular pulley binder for high-capacity silicon anodes of lithium ion batteries in Science on July 20.
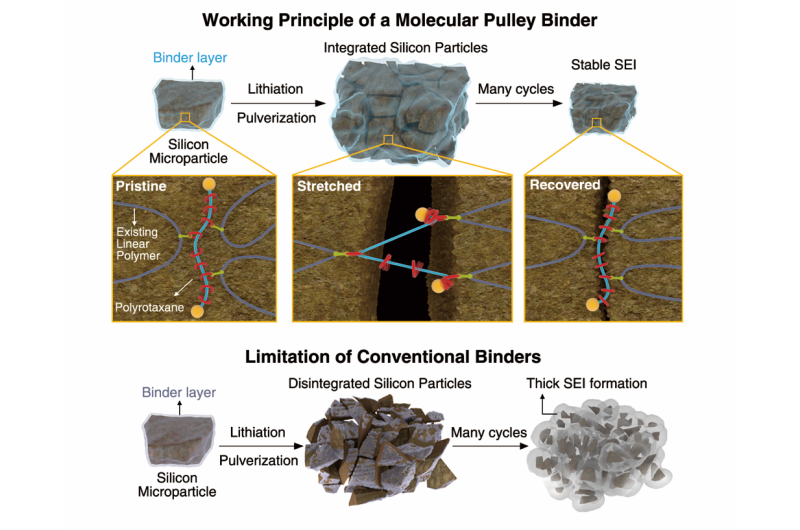
The KAIST team integrated molecular pulleys, called polyrotaxanes, into a battery electrode binder, a polymer included in battery electrodes to attach the electrodes onto metallic substrates. In a polyrotaxane, rings are threaded into a polymer backbone and can freely move along the backbone.
The free moving of the rings in polyrotaxanes can follow the volume changes of the silicon particles. The rings' sliding motion can efficiently hold Si particles without disintegration during their continuous volume change. It is remarkable that even pulverized silicon particles can remain coalesced because of the high elasticity of the polyrotaxane binder. The functionality of the new binders is in sharp contrast with existing binders (usually simple linear polymers) with limited elasticity, since existing binders are not capable of holding pulverized particles firmly. Previous binders allowed pulverized particles to scatter, and the silicon electrode thus degrades and loses its capacity.
The authors note, "This is a good example of the importance of fundamental research. Polyrotaxane received the Nobel Prize last year based on the concept of a 'mechanical bond.' This is a newly identified concept, and can be added to classical chemical bonds in chemistry, such as covalent, ionic, coordination and metallic bonds. The long fundamental study is now expanding in an unexpected direction that addresses longstanding challenges in battery technology."
The authors also mention that they are currently working with a major battery maker to get their molecular pulleys integrated into real battery products.
Sir Fraser Stoddart of Northwestern University, the 2016 Noble Laureate in Chemistry, says, "Mechanical bonds have come to the rescue for the first time in an energy storage context. The KAIST team's ingenious use of mechanical bonds in slide-ring polyrotaxanes—based on polyethylene glycol threaded with functionalized alpha-cyclodextrin rings—marks a breakthrough in the performance of marketable lithium-ion batteries. This important technological advance provides yet more evidence that when pulley-like polymers carrying mechanical bonds displace conventional materials based on chemical bonds alone, the unique influence of this physical bond on the properties of materials and the performance of devices can be profound and game-changing."
More information: S. Choi el al., "Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries," Science (2017). science.sciencemag.org/cgi/doi … 1126/science.aal4373
Journal information: Science