Protective shells may boost silicon lithium-ion batteries
Imagine a cell a phone that charges in less than an hour and lasts for three to four days or an electric car that runs for hundreds of miles before needing to be plugged in.
Researchers at the U.S. Department of Energy's Argonne National Laboratory are working to make this dream a reality by developing lithium-ion batteries containing silicon-based materials. The most commonly used commercial lithium-ion batteries are graphite-based, but scientists are becoming increasingly interested in silicon because it can store roughly 10 times more lithium than graphite.
"When we talk about batteries, we talk in terms of the amount of energy that can be stored," said Daniel Abraham, materials scientist in Argonne's Chemical Sciences and Engineering Division. "Silicon-based batteries could double or even triple the energy stored in conventional batteries, which would greatly benefit the consumer electronics market and the automotive industry."
There's just one problem: current batteries based on silicon materials don't last long.
The problem lies in the battery's chemistry. The electrolyte inside the battery transports lithium ions back and forth between positive and negative electrodes as the battery charges and discharges. The positive electrode contains a lithium-bearing compound, while the negative electrode contains materials such as graphite or silicon.
Lithium ions react with the negative electrode to form a new compound, causing the electrode to expand, while the electrolyte produces a protective coating called the solid electrolyte interphase.
"The ideal solid electrolyte interphase should halt the reaction between the electrode and electrolyte, while allowing the lithium to come through," said Ilya Shkrob, a chemist in the Chemical Sciences and Engineering Division.
But the coating also needs to expand and contract with the electrode, or else it will crack and the battery won't work.
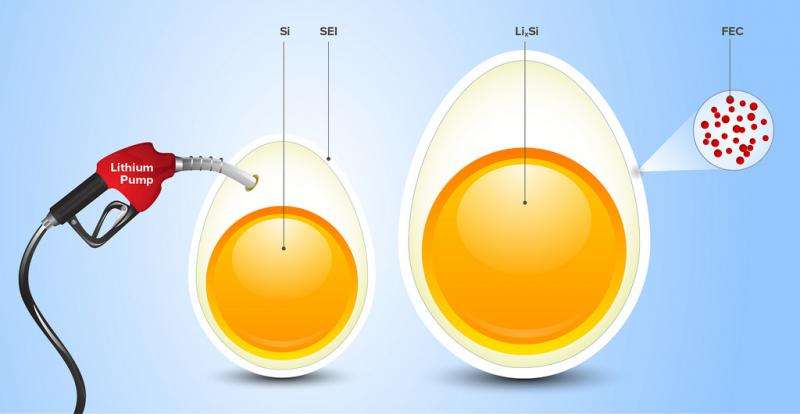
Shkrob compared this phenomenon to an imaginary experiment – pumping air into an egg. The egg starts to expand and the shell cracks. The shell cracks again when the air is released and the egg returns to its original size.
"When the protective layer cracks, the electrode surface reacts and consumes the electrolyte," Shkrob said. "If the electrolyte is completely consumed, then the battery won't work."
In today's graphite-based lithium-ion batteries, the electrode expands about 10 percent – a small enough change that cracks in the coating aren't an issue.
But the electrode in a silicon-based lithium-ion battery expands up to 300 percent. These batteries need a different electrolyte in order to produce an elastic shell.
The researchers found that when fluorine is added to ethylene carbonate, the resulting electrolyte forms a coating that can stretch and accommodate the volume changes in the electrode.
"The fluorine is what gives this molecule its special power," Shkrob said. "Fluoroethylene carbonate forms a protective layer that acts like rubber. Our achievement is to show how a small change in molecular structure can completely change the properties of the protective shell."
However, Abraham noted that the team has not completely solved the electrolyte problem. "There may be other compounds that produce a rubber-like coating more efficiently than the fluoroethylene carbonate molecules," he added.
"It's the idea that's important – an egg shell versus rubber," Abraham said. "Solving the electrolyte problem is an important step towards commercializing silicon-based lithium-ion batteries, which will vastly increase the amounts of energy we can store."
More information: "What Makes Fluoroethylene Carbonate Different?" J. Phys. Chem. C, 2015, 119 (27), pp 14954–14964 DOI: 10.1021/acs.jpcc.5b03591
Provided by Argonne National Laboratory