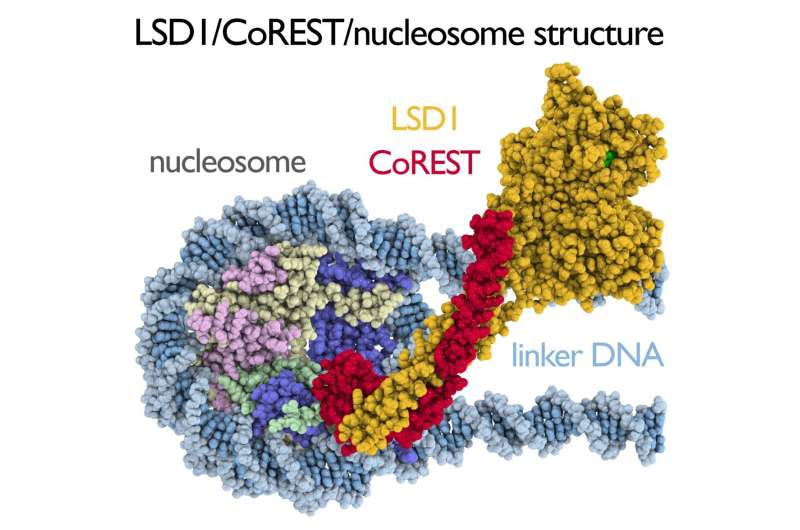
A new atomic model for the LSD1/CoREST histone methylase in complex with its nucleosome substrate could provide important insight into how cells regulate their genes. Credit: Song Tan, Penn State
New images of an enzyme in action as it interacts with the chromosome could provide important insight into how cells—including cancer cells—regulate their genes.
The enzyme, LSD1, can "turn off" gene expression by removing chemical flags (methyl groups) from the nucleosome—tightly packed units of DNA and protein in chromosomes. This LSD1 histone demethylase is over-expressed in multiple cancer types, resulting in disruption to normal cell development, and the new structure could inform therapeutic interventions that target the enzyme.
A paper by Penn State researchers describing the crystal structure of the LSD1-nucleosome complex publishes in the June 4 issue of the journal Molecular Cell and also explains the previously unclear but important role of a separate accessory protein, CoREST.
"While previous studies have imaged LSD1 and CoREST with only a small portion of the nucleosome—a histone tail—we managed to image these proteins with the entire nucleosome core particle to better understand their role in gene regulation," said Song Tan, professor of biochemistry and molecular biology, director of the Center for Eukaryotic Gene Regulation at Penn State, and leader of the research team. "We knew that LSD1 required the accessory protein CoREST in order to work on the nucleosome, but we didn't know what CoREST was doing. When we saw the structure, we were initially surprised but then realized everything made sense."
The research team found that LSD1 unexpectedly binds outside of the core of the nucleosome, where most activities on the nucleosome take place. LSD1 instead binds to DNA that extends away from the core, and CoREST acts as an intermediary, binding to both the nucleosome core and to LSD1.
"This was astonishing because all other enzymes that have been imaged bind directly to the nucleosome core where they do their job," said Tan.
The structure also explains how the CoREST accessory protein enables LSD1 to work on nucleosomes. The researchers believe that the LSD1-CoREST system could serve as a model for how other accessory proteins target chromatin enzymes to nucleosomes.
"LSD1 is like a pilot who needs a navigator to know where to go," said Tan. "The CoREST accessory protein acts as the navigator to target LSD1 to nucleosomes."
The research team also investigated a version of the LSD1 enzyme with a mutation believed to render it inactive. Such inactive mutants have been used to test the effects of eliminating LSD1's activity in cells. However, the team found that the mutant is actually active in the presence of the nucleosome.
"The LSD1 K661A mutant was presumed to be inactive based on assays using just a histone tail portion of the nucleosome," said Tan. "However, when we use the entire nucleosome core particle, which is more representative of what actually exists in the cell, we see that the mutant is very active. This means the conclusions of studies which used LSD1 K661A as an inactive mutant will need to be reassessed since the enzyme's activity was not actually eliminated."
More information: Sang-Ah Kim et al, Crystal Structure of the LSD1/CoREST Histone Demethylase Bound to Its Nucleosome Substrate, Molecular Cell (2020). DOI: 10.1016/j.molcel.2020.04.019
Journal information: Molecular Cell
Provided by Pennsylvania State University