This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New structures offer insight into how a bacterial motor powers bacterial chemotaxis, a key infectious process
Bacteria existed for millennia before humans and have been infecting us from the beginning. Although we can treat infections through pharmaceuticals, bacteria continue to become resistant to treatment thanks to their rapid evolution. Bacterial infections remain a leading cause of morbidity and mortality in 2024, resulting in nearly eight million annual deaths globally.
One key characteristic shared by all infectious bacteria is called chemotaxis. Chemotaxis is a versatile process that allows bacteria to swim toward energy-rich molecules, find preferred niches for infection, avoid harmful species, change speeds, and fully stop to form biofilms. Chemotaxis is also essential for virulence in animals and a potential target for new therapeutics. But first, the process itself needs to be better understood.
The lab of Tina Iverson, Louise B. McGavock Professor and professor of pharmacology, in collaboration with researchers at the University of California, San Francisco; Stanford University; and The Weizmann Institute of Science in Israel have published new work in Nature Microbiology, providing new insights on chemotaxis.
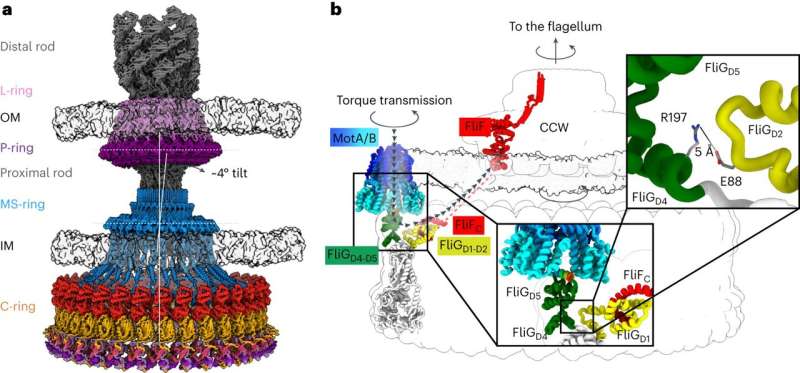
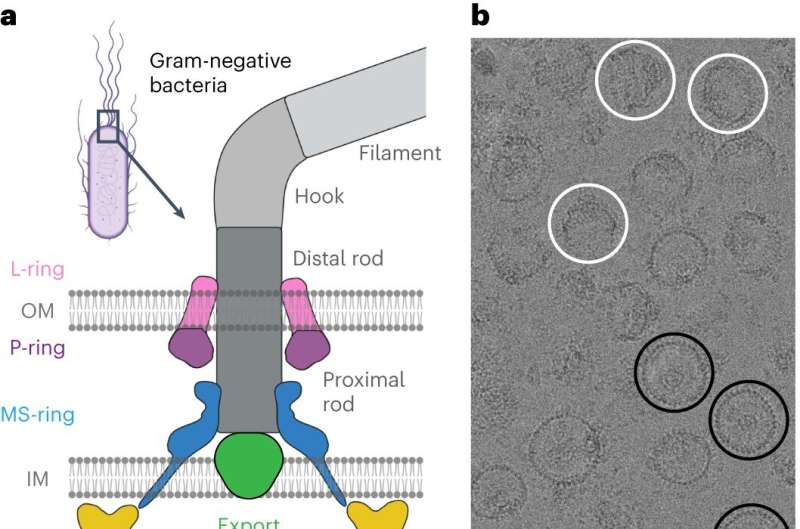
Chemotaxis requires a small motor to turn a flagellum—a hairlike appendage on bacteria that spins to provide propulsion, like a boat motor. Rotating the flagellum clockwise or counterclockwise at different rates allows bacteria to move toward or away from different stimuli. Current research hasn't come up with an agreed-upon architecture of the central components of the motor that powers the flagellum, which has hindered researchers' understanding of and ability to target chemotaxis with drugs.
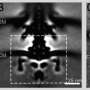
The current work, spearheaded by senior research associate in the Iverson lab Prashant Singh, puts forth new information about how a motor component called a switch reverses rotation and transmits torque to the flagellum.
To do this, the researchers looked at Salmonella enterica, a bacterium responsible for approximately 60,000 deaths globally per year, as a model. After isolating and purifying S. enterica motors stabilized in different swimming configurations, the collaborators leveraged the power of Vanderbilt's Titan Krios, a $10M cryo-electron microscope acquired by the School of Medicine Basic Sciences that was made available through the Center for Structural Biology's Cryo-EM Facility.
The structures provided the researchers with information about how the bacterial motor powers clockwise and counterclockwise rotation of the flagellum, which allows a bacterium to swim straight or switch directions while swimming. It also helped them understand how proteins bind to the motor to help regulate bacterial movement.
These results are applicable to a broad range of infections. For instance, the Salmonella chemotaxis machinery is nearly identical to that of Escherichia coli, which is responsible for over 250,000 infections per year in the U.S. alone. Because chemotaxis is required for infection, selectively disrupting the interactions that allow pathogens to form a reservoir within an organism can help to prevent recurrent infections without impacting the normal microbiome.
The Iverson lab is now working to identify how an expanded range of different protein partners bind to the flagellar motor during chemotaxis, and hope that this will lead to ways to disrupt chemotaxis during infection.
More information: Prashant K. Singh et al, CryoEM structures reveal how the bacterial flagellum rotates and switches direction, Nature Microbiology (2024). DOI: 10.1038/s41564-024-01674-1
Journal information: Nature Microbiology
Provided by Vanderbilt University