Light-induced bifunctional rhodium catalysis for decarbonylation. Credit: MIN Xiangting
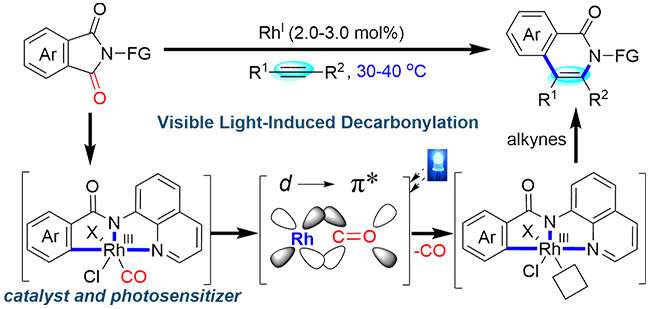
Carbonyl groups are ubiquitous in pharmaceuticals, natural products, and agricultural chemicals, especially amides. Transition metal-catalyzed decarbonylation offers a distinct synthetic strategy for new chemical bond formation and carbonyl groups transformation. However, the π-backbonding between CO π* orbitals and metal center d-orbitals impedes ligand dissociation to regenerate catalyst under mild condition.
Recently, a team led by Prof. CHEN Qing-an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a visible light-induced bifunctional rhodium catalysis for decarbonylative coupling of imides with alkynes under ambient conditions.
This study was published in Angew. Chem. Int. Ed. on Sept. 29.
Initial mechanistic studies suggested that rhodium complex simultaneously served as the catalytic center and photosensitizer for decarbonylation. Under visible light irradiation, it generated the excited state Rh-complex where the transfer of an electron from the π-backbonding Rh-CO orbital into an antibonding orbital decreased the bond dissociation energy of the Rh-CO bond.
This visible light-promoted catalytic decarbonylation strategy offers new opportunities for carbonyl groups transformation.
More information: Xiang-Ting Min et al. Visible Light‐Induced Bifunctional Rhodium Catalysis for Decarbonylative Coupling of Imides with Alkynes, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202010782
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences