Researchers glean insights into how enzymes achieve specific reactions
Catalytic dioxygen activation and selective oxidative cleavage of C–C bonds have become the research hotspots in the field of chemistry due to their great application value in organic synthesis and industrial production.
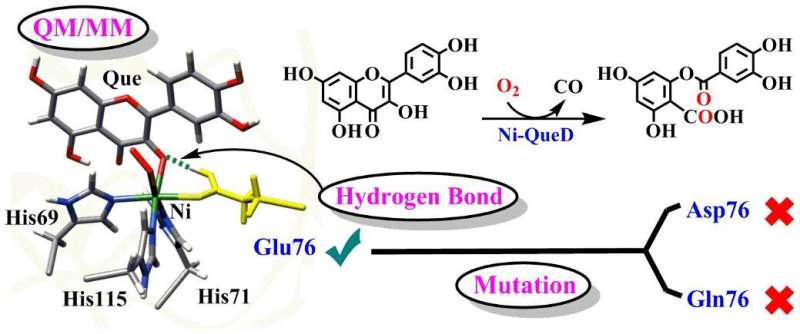
Quercetin 2,4-dioxygenases (QueDs), as a typical metalloenzymein biological system, can efficiently activate dioxygen and selectively catalyze C–C bond oxidative cleavage of organic flavonol substrates under mild conditions. Although studies on QueDs have been carried out for decades, the detailed catalytic mechanisms of QueDs, especially the roles of the Glu76 residue at active site, are still under debate.
In a study published in the Journal of Catalysis, a group led by Prof. LI Chunsen from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences reported the detailed mechanisms of reactions catalyzed by wild-type nickel-dependent quercetin 2,4-dioxygenase (Ni-QueD) and its Glu76Asp and Glu76Gln mutants by using combined MD simulations and QM/MM calculations, and they revealed the critical role of Glu76 residue in steering the reactivity of Ni-QueD.
The researchers found that the conserved nickel-ligating Glu76 residue in the deprotonated form is essential for initiating the catalytic reaction by proton coupled electron transfer process.
The generated and protonated Glu76 promotes the subsequent reaction by regulating hydrogen-bonding (H-bonding) interaction with the carbonyl groups of quercetin.
Investigations of Glu76Gln and Glu76Asp mutants show that mutation of Glu76 suppresses such H-bonding interaction and results in the lower catalytic activity observed experimentally.
This study provides not only useful information about the mechanisms of reactions catalyzed by metal ion-dependent QueDs, but also insights into how enzymes achieve specific reactions by using the H-bonding interaction from the metal center-ligating residues.
More information: Xueyuan Yan et al. Mechanistic insights into the crucial roles of Glu76 residue in nickel-dependent quercetin 2,4-dioxygenase for quercetin oxidative degradation, Journal of Catalysis (2020). DOI: 10.1016/j.jcat.2020.04.016
Provided by Chinese Academy of Sciences