Sustainable iron catalysis enables controllable alkene borylation

National University of Singapore chemists have unlocked a way to achieve site-selective borylation of alkenes using earth-abundant iron-based catalysts to facilitate synthesis of high-value chemicals.
Owing to their numerous desirable attributes, boron-containing molecules play a key role in drug discovery and serve as indispensable precursors to diverse chemical products ranging from pharmaceuticals to polymeric materials. In this context, an attractive approach to access aliphatic organoboron compounds involves catalytic hydroboration or protoboration of carbon-carbon (C-C) double bonds, reactions in which hydrogen and boron are both added to an alkene. A modern variation of this process involves remote borylation, whereby boron is inserted distant from the initial reactive site. However, existing methods often lead to borylations at the terminus or at activated positions geminal (α) to functional units. Installing the coveted boryl group at unactivated positions is a formidable challenge.
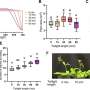
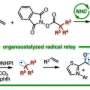
A research team led by Prof KOH Ming Joo, from the Department of Chemistry, NUS has developed a new catalytic transformation known as remote protoboration. In this system, a single iron-based complex promotes alkene isomerisation followed by site-selective proto-boryl addition to the C=C bond. Thus, boron can be installed at typically less-activated positions vicinal (β) to common functional groups. The protocol has significant scope and utility in the synthesis of medicinally relevant compounds.
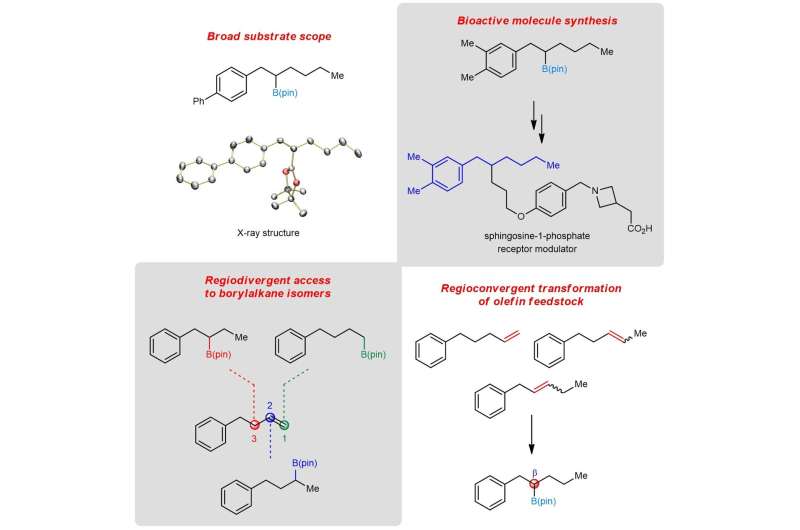
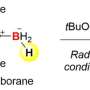
Detailed mechanistic studies revealed that the success of remote protoboration lies in the in situ-generation of two catalytic organoiron species (iron-hydride and iron-boryl) that operate in synergy. Leveraging on these insights, the team demonstrated that mixtures of alkene regioisomers (in unrefined chemical feedstock) can be converted to a single product, and selective access to borylalkane regioisomers from one alkene substrate can be accomplished.
Prof Koh said, "The two organoiron catalytic intermediates possess different reactivity profiles, allowing them to co-exist and react in a selective fashion. A key controlling factor is the amount of proton source present, either adventitiously generated in situ or exogenously added to the reaction mixture. This helps to tune the relative rates of alkene isomerization and protoboration."
"This discovery has tremendous implications beyond remote protoboration. If we can precisely manipulate the position of alkene shifts and subsequent proto-boryl insertion, we can potentially install boron at any desired location of an organic molecule. The insights gained from our studies are expected to advance general efforts towards this ultimate goal," added Prof Koh.
The research team plans to apply the new concepts to address other challenges pertaining to site-selective functionalisations of organic molecules for the synthesis of important compounds of interest.
More information: Xiaolong Yu et al. Site-selective alkene borylation enabled by synergistic hydrometallation and borometallation, Nature Catalysis (2020). DOI: 10.1038/s41929-020-0470-9
Journal information: Nature Catalysis
Provided by National University of Singapore