This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Synthesis of two new carbides provides perspective on how complex carbon structures could exist on other planets
Researchers at the University of Bayreuth have gained new insights in the field of high-pressure carbon chemistry: They synthesized two new carbides—compounds of carbon and another chemical element—with unique structures. The results may provide an unexpected explanation of the wide spread of polycyclic aromatic hydrocarbons in the universe. The research is published in the journal Nature Communications.
Carbides are compounds of carbon and another chemical element. The newly synthesized carbides resemble metal-organic-like compounds and can offer new insight into the behavior of complex carbon structures under extremely high pressures and high temperatures.
The possible existence or formation of such compounds at conditions of planets' interiors may have important implications for geosciences and astrobiology, as they could be the origin of hydrocarbons and could play a role in the origin of life.
Under the leadership of Prof. Dr. Leonid Dubrovinsky from the Bavarian Geoinstitute and Prof. Dr. Natalia Dubrovinskaia from the Laboratory of Crystallography at the University of Bayreuth, the research into the new carbon compounds reveals they have structural elements similar to those of complex organic molecules, but are deprotonated (i.e., do not contain hydrogen).
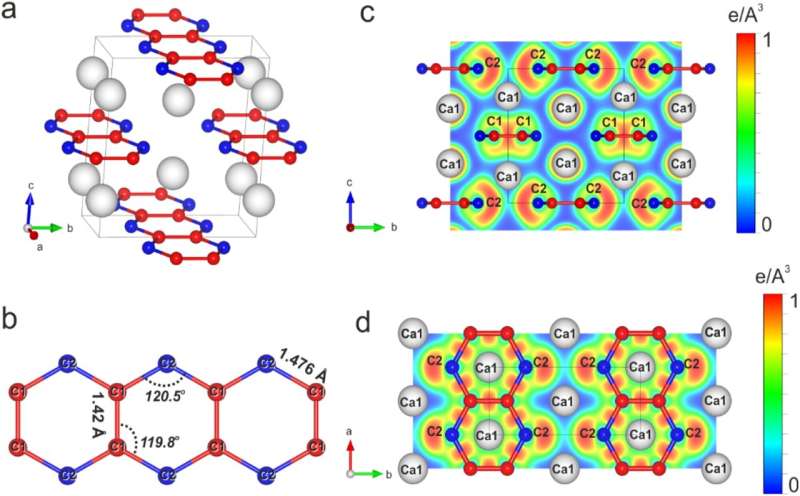
To accomplish this, the researchers used diamond anvil cells that compressed the tiny calcium carbide crystals to pressures in the three-digit gigapascal range and simultaneously heated them to temperatures of about 3000°C. These conditions correspond to those at a depth of 2.900 km in the Earth's interior. The change in pressure and temperature caused the calcium carbide to form two new carbides: high-pressure polymorph of CaC2 and Ca3C7.
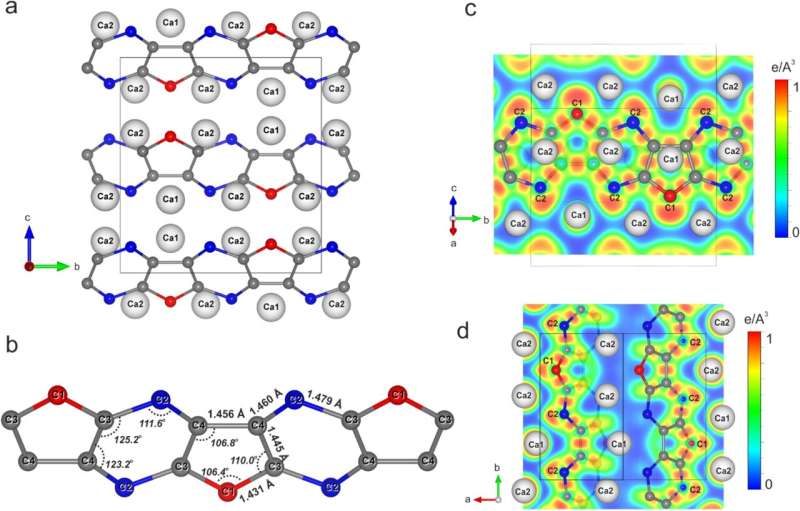
Although the high-pressure polymorph of CaC2 has the same chemical composition as the starting material, it differs from it in the spatial arrangement of the atoms and in its chemical properties. The polymorph possesses carbon chains that can exist under conditions that far exceed those known for the existence of conventional organic compounds.
The formation of such compounds under the conditions in the interior of planets could even have played a role in the origin of life, because they could be the origin of hydrocarbons.
The compound with the chemical formula Ca3C7 has never been observed before, so that its synthesis and structure elucidation represent a significant step forward in understanding the behavior of carbon-based materials under extreme conditions.
Prof. Dr. Leonid Dubrovinsky, the lead researcher of the study, explained, "Our findings not only expand the boundaries of known carbon chemistry but also provide a new perspective on how complex carbon structures could exist in the deep Earth and potentially in other planetary bodies."
"The similarities between these high-pressure carbides and deprotonated metal-organic compounds open up exciting possibilities for designing novel materials with unique electronic, magnetic, and optical properties," added Prof. Dr. Natalia Dubrovinskaia.
More information: Saiana Khandarkhaeva et al, Extending carbon chemistry at high-pressure by synthesis of CaC2 and Ca3C7 with deprotonated polyacene- and para-poly(indenoindene)-like nanoribbons, Nature Communications (2024). DOI: 10.1038/s41467-024-47138-2
Journal information: Nature Communications
Provided by Bayreuth University