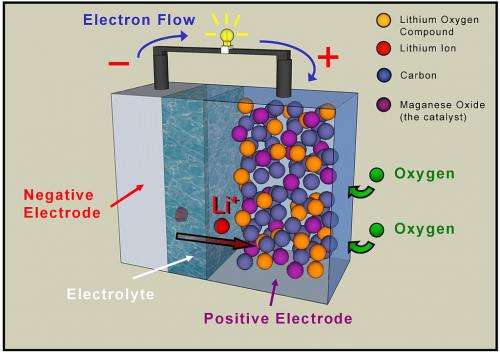
Lithium-air batteries are particularly appealing to researchers because they have a significantly higher theoretical capacity than conventional lithium-ion batteries.
The creation of the next generation of batteries depends on finding materials that provide greater storage capacity. One variety, known as lithium-air (Li-air) batteries, are particularly appealing to researchers because they have a significantly higher theoretical capacity than conventional lithium-ion batteries.
Li-air battery development is still in its infancy, however, and like most new technologies, it faces many challenges. One of these challenges involves the transfer of charge to the anode, which along with the cathode and electrolyte, is one of the three principal components of a battery.
In a new study, electrochemist Di-Jia (D.J.) Liu and his colleagues at the U.S. Department of Energy's Argonne National Laboratory studied anode behavior inside lithium-air batteries during the battery's cycling.
By using high-energy, focused X-ray beams provided by Argonne's Advanced Photon Source (APS), Liu and his team were able to non-destructively peer inside an operating battery to study the changes in the anode microstructure. They saw the formation of a thin solid coating of lithium hydroxide (LiOH), which continued to grow at the expense of lithium metal until the metal was totally converted to hydroxide and shut down the operation.
"This was the kind of question that everyone wanted to know but was afraid or didn't know how to ask," Liu said. "Nearly all the literature on Li-air batteries so far focused on the chemical processes at cathode while assuming the anode is completely reversible. But now we know that this is not the case."
The team's investigation did not stop there. Since lithium hydroxide is not an ion or an electron-conducting material, it remained a mystery to understand how the coating does not stop the battery from cycling as soon as it forms. Using a 3-dimensional micro-tomography technique at the APS, Liu and his collaborators were able to perform a "CAT scan" of the hydroxide coating and found numerous microscopic tunnels connecting to the metallic lithium at the anode to the rest of the battery.
"These tunnels serve as ion-conducting channels that shuttle lithium ions between the anode and cathode," Liu said. "They sustained the Li-air battery operation but did not stop the anode decay."
Liu believes that one major cause of the lithium hydroxide problem could involve the breakdown of the battery's electrolyte – the medium that transports lithium ions between the two electrodes. Decomposition of the electrolyte has the potential to form water near the cathode, which could then migrate to the anode and react with the metallic lithium.
However, the Argonne experiment didn't reveal any lithium hydroxide near the cathode, which should have been present if water were formed by the breakdown of the electrolyte. "I think that we generated as many questions as answers through this study, which is the exciting part of science," he said.
This work was performed by an all Argonne team including beamline scientists John Okasinski, Peter Kenesei, Jonathan Almer and chemistry postdocs Jianglan Shui and Dan Zhao. It was supported by the U.S. Department of Energy's Office of Science and Argonne's Grand Challenge Program. The work was published in the August 9 issue of Nature Communications.
Journal information: Nature Communications
Provided by Argonne National Laboratory