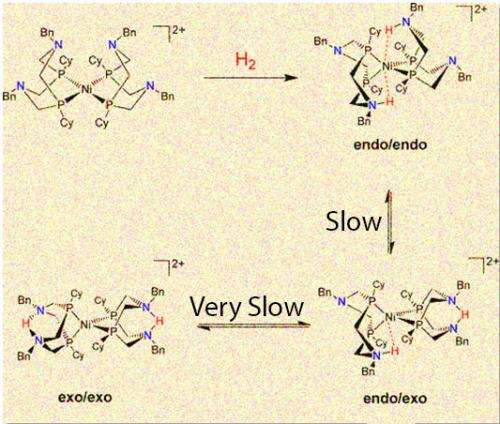
While one configuration (endo/endo) of a popular nickel catalyst can produce thousands of hydrogen molecules a second, the other forms that place the proton farther from the center are slower and less efficient.
(Phys.org)—Proton delivery and removal determines if a well-studied catalyst takes its highly productive form or twists into a less useful structure, according to scientists at Pacific Northwest National Laboratory. The catalyst takes two protons and forms molecular hydrogen, or it can split the hydrogen. The team showed that the most productive isomer, endo/endo, has the key nitrogen-hydrogen bonds pushed close to the nickel center. If the catalyst is in the endo/endo form, the reaction occurs in a fraction of a second. If the catalyst is stuck in another form, the reaction takes days to complete.
"When we started on the research, there was the belief that breaking or forming hydrogen was the crucial step," said Dr. Simone Raugei, a PNNL theoretician. "It isn't. It is putting protons in the right spot on the catalyst. Once you have them in the right spot, everything goes very quickly."
The fundamental chemical questions around how protons move underlie a host of energy challenges, including fuels and fertilizer production. Agricultural waste and other fuel feedstocks are packed with oxygen that needs to be replaced with hydrogen. Adding hydrogen to nitrogen to create ammonia for fertilizer uses about 1% of the world's energy. Improving these reactions and those that result in the widespread use of fuel cells requires understanding the basics of how protons move.
"The catalyst we studied is the fastest of its type with hydrogen, but it still isn't fast enough to put in a fuel cell and drive down the road," said Dr. Wendy Shaw, a biophysical chemist at PNNL. "To get the catalysts to achieve their full potential, we need to understand all of the bottlenecks and how to overcome them."
The researchers began with a well-known nickel-based catalyst. The catalyst is dissolved in a liquid, which is bubbled with hydrogen gas. The catalyst pulls the hydrogen apart. Run under slightly different conditions, the catalyst can take two protons and create molecular hydrogen or H2.
The catalyst has four "floppy" arms or ligands around the nickel center. These nitrogen-containing ligands twist into three different isomers, which are different arrangements of the same atoms. The isomers differ in the position of a nitrogen-hydrogen bond in respect to the nickel center. The isomer known as endo/endo is the fastest. The isomers exo/exo and endo/exo are much slower, with the exo/exo form taking days, compared to endo/endo's fraction of a second.
The team set out to see how the protons move on the catalyst's ligands between each of the different isomers. Shaw and Dr. Molly O'Hagan conducted experiments using nuclear magnetic resonance spectroscopy to examine the three isomers. The theoreticians determined the barriers that kept the catalyst in the less-productive isomer forms. They used computing resources at the Environmental Molecular Sciences Laboratory and the National Energy Research Scientific Computing Center, along with Jaguar at Oak Ridge National Laboratory through a prestigious INCITE award supported by the Department of Energy's Office of Science.
The team found that initially the protons preferred to be positioned away from the nickel. Getting them from this place to a position near the nickel takes several chemical steps, requiring energy and slowing down the catalyst. To run the reaction in the opposite direction, they found it is difficult to remove protons when they are next to nickel.
"Proton delivery is key to catalysis. If you want to improve these and other catalytic platforms, you have to improve proton delivery," said Raugei.
The team suspects the catalyst could be 10 times faster if it would avoid the two distracting isomers. With the demand from biofuels and fuel cells on the rise, this research is ongoing at the Center for Molecular Electrocatalysis at PNNL.
More information: O'Hagan, M. et al. Proton Delivery and Removal in [Ni(PR2NR′2)2]2+ Hydrogen Production and Oxidation Catalysts. Journal of the American Chemical Society 134, 19409-19424. DOI: 10.1021/ja307413x
Journal information: Journal of the American Chemical Society
Provided by Pacific Northwest National Laboratory