Credit: AI-generated image (disclaimer)
Although it is widely used in the food, cosmetic, and pharmaceutical industries worldwide, the breakdown of carminic acid (a natural red dye extracted from insects) in nature has remained unclear. Through a combination of biochemical and structural analyses, researchers from Japan have now provided insight into this puzzle.
In a study published on October 5 in Proceedings of the National Academy of Sciences, a research team led by the University of Tsukuba has discovered a soil microorganism possessing an enzyme that initiates the first step in breaking down carminic acid.
Carminic acid is synethesized by cochineal scale insects, which are parasites of Cacti. This compound is a natural C-glycoside—a unique compound in which a sugar is bound to a non-sugar (or aglycone) molecular group via a carbon-carbon bond. Although enzymes have been previously identified in intestinal microorganisms that initiate the breakdown of C-glycosides, the details of how this occurs in nature is not fully understood. Clarifying the enzyme structure and mechanistic pathway were key points that the research team aimed to address.
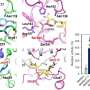
"Carminic acid is very important as a natural 'red dye' all over the world, but its metabolic pathway in nature has never been identified," explains the senior author of the study, Professor Michihiko Kobayashi. "We screened and isolated a carminic acid-catabolizing microorganism from soil samples and found a flavin adenine dinucleotide (FAD)-dependent enzyme, that we termed CarA, that catalyzes the first step of C-glycoside-metabolism by oxidizing the C3 position of the sugar moiety."
The researchers collected soil samples from around the University of Tsukuba and isolated microorganisms that could grow on an agar plate with only carminic acid as the carbon source. The team focused on one bacterial strain, named 5-2b, because the red coloring from carminic acid disappeared around colonies from this specific bacterial strain, strongly suggesting degradation of the natural red dye.
Interestingly, although a similar overall pathway for the breakdown of carminic acid was demonstrated for both intestinal and soil microorganisms, the enzyme catalyzing the initial step completely differed between the two strains. "Our investigations suggest that the differences in the carminic acid metabolizing enzyme between soil and intestinal bacteria possibly arise from the adaptation to aerobic and anaerobic environments, respectively, because CarA needs oxygen for its enzymatic activity," explains the lead author Professor Takuto Kumano.
Furthermore, the authors found that CarA could initiate the breakdown of some other types of glycosides as well, although not as well as for carminic acid.
"Our findings now provide insight into the previously unknown biogeochemical circulation of carminic acid and C-glycosides in nature," says Professor Kobayashi. "Now that we have a crystal structure of a homolog of CarA, the next essential step is obtaining a structure that contains a substrate."
More information: Takuto Kumano et al, FAD-dependent C-glycoside–metabolizing enzymes in microorganisms: Screening, characterization, and crystal structure analysis, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2106580118
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Tsukuba