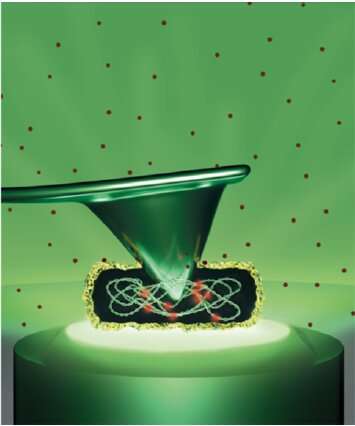
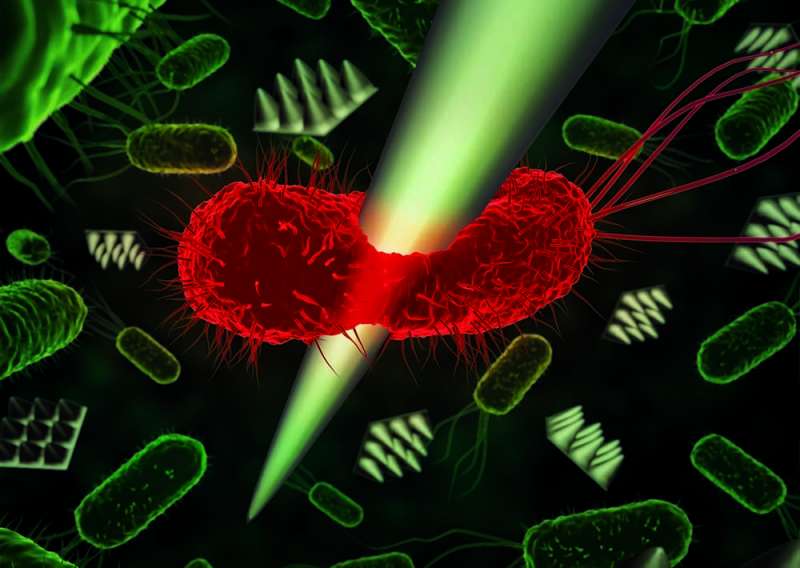
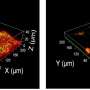
Simultaneous AFM nanoindentation and fluorescence imaging of E. coli. Credit: Patricia Bondía
Antibiotic resistance is one of the biggest threats to global health and food security today according to the World Health Organization. This process occurs naturally, but misuse of antibiotics in humans and animals is accelerating it. In this context, mechano-bactericidal materials emerge as a promising strategy to tackle bacterial resistance to antibiotics.
Mechano-bactericidal materials pose a surface structure landscape hostile to bacteria, and researchers are making efforts to understand the physical interactions between bacteria and these antimicrobial nanomaterials. By revealing all details of the interaction, such as the force necessary to kill bacteria, a new and improved generation of mechano-bactericidal materials can be designed.
A team of researchers at IMDEA Nanociencia have quantitatively studied the forces necessary to exert critical damage on a single E. coli bacterium under physiological conditions. In their work, published in ACS Applied Materials and Interfaces, researchers led by Dr. Flors use AFM (atomic force microscopy) nanoindentation to puncture the bacterium cell wall, and observe bacterial death in real time by using a fluorescent viability marker. They conclude that a force of about 20 nN is necessary to break the cell wall in E. coli.
Consecutive indentations at a low force but with no cell wall rupture produced a measurable 'fatigue' effect on bacterial physiology. The fatigue was determined by following the oscillation period of the Min system, a protein complex that helps determining the cell division site in bacteria, by fluorescence microscopy. The observed fatigue effect is consistent with the antibacterial properties of colloidal nanomaterials by the accumulative action of many low-force collisions.
Fluorescent bacterium after it has been mechanically damaged by a sharp object. Credit: Patricia Bondía
The results provide a detailed view, unknown till now, of the interaction between bacteria and high aspect ratio mechano-bactericidal nanomaterials, and contribute to the emerging field of mechanomicrobiology. "Our work showcases how the development of advanced microscopy techniques can play a part to quantitatively understand the interactions between bacteria and nanomaterials," Dr. Flors says.
More information: Adrián del Valle et al. Mechanically Induced Bacterial Death Imaged in Real Time: A Simultaneous Nanoindentation and Fluorescence Microscopy Study, ACS Applied Materials & Interfaces (2020). DOI: 10.1021/acsami.0c08184
Journal information: ACS Applied Materials and Interfaces
Provided by IMDEA Nanociencia