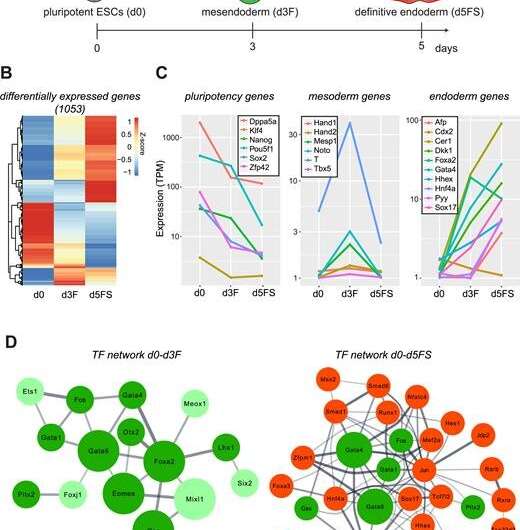
An in vitro differentiation system modelling early endoderm differentiation. (A) Endoderm differentiation of ESCs is triggered by Wnt3A/Activin A treatment. Mesendoderm (Foxa2-Venuspos; Sox17-Cherryneg) and endoderm (Foxa2-Venuspos; Sox17-Cherrypos) cells can be isolated by FACS. (B) Heat map showing z-scores of the expression levels of the 1053 differentially expressed genes between pluripotent ESCs (d0), mesendoderm (d3F) and endoderm (d5FS) cells (Padj < 0.05, fold change > 2; n = 2 for each condition). (C) Average expression levels of selected marker genes for pluripotent, mesoderm and endoderm cells in the in vitro differentiated ESCs (TPM – Transcripts Per Kilobase Million; n = 2 for each condition). Nucleic Acids Research (2019). DOI: 10.1093/nar/gkz627
Transcription factors control gene activation in cells. By binding to specific segments of DNA, they enable the blueprints that code for cellular proteins to be produced. But how are such factors themselves regulated?
In the cell nucleus, the long DNA molecules that comprise the hereditary material are tightly folded into a highly condensed form. This makes it possible for the 2 m of DNA found in every nucleus in the human body to be accommodated at all. Each double-stranded DNA is wrapped around millions of protein complexes composed of 'histones," and these 'nucleosomes' are in turn connected by linker histones. The resulting compactified fraction of the chromosomal DNA is referred to as 'chromatin."
However, in order to grow, differentiate and respond to changing conditions, every cell must be able to selectively activate the genes which provide the blueprints for synthesis of the proteins that it needs. This means that specific sets of genes within the tightly packaged chromatin must be made accessible to the enzymes responsible for 'transcription' of these blueprints. How do cells accomplish this task? How is the packaging of chromatin locally modified to enable transcription of precisely those segments of the DNA where these genes reside?
A specialized class of proteins, known as pioneer transcription factors (PTFs), is responsible for opening up the chromatin at particular sites. They do so by displacing nucleosomes from regions in which the DNA is tightly packed. PTFs thus serve as gatekeepers, which enable the transcriptional machinery to interact with the genes whose protein products are currently required. This of course raises the question of how PTFs actually clear the way and, perhaps even more important, how their actions are controlled. A team led by Professor Gunnar Schotta and Dr. Filippo Cernilogar at LMU's Biomedical Center, together with Professor Heiko Lickert of the Helmholtz Zentrum München, has now elucidated some aspects of this complex riddle.
The researchers addressed these issues in laboratory experiments designed to uncover the mechanisms that enable embryonic stem cells to generate endodermal cells during mouse embryogenesis. These cells subsequently form inner organs of the body. It is known that a PTF named Foxa2 is involved in the process. Foxa2 interacts with specific binding sites in the DNA. This interaction can result in displacement of nucleosomes which makes DNA sequences that code for proteins essential for the development of endodermal tissues accessible for binding by other proteins. Indeed, Schotta and his colleagues have now shown that other TFs in addition to Foxa2 play an essential role in this process.
Furthermore, it appears as if Foxa2's ability to recognize the binding sites that are important for the early development of endoderm is dependent on second layer of regulation. For gene regulation is mediated not only by binding of proteins to regulatory nucleotide sequences in the DNA. It can also involve the recognition of chemical tags attached to the nucleotide subunits of the DNA or to nucleosomal histones. This latter type of control is referred to as 'epigenetic," because the modifications do not change the coding capacity of the DNA sequence itself. Thus attachment of epigenetic tags to histones also serves to modulate the accessibility of nucleosomal DNA. Indeed, Schotta's team has now shown that the density of epigenetic tags determines whether or not Foxa2 can bind and open up condensed chromatin. "These results reveal important details of the process by which a network of transcription factors can control the activity of key genes," he says.
More information: Filippo M Cernilogar et al. Pre-marked chromatin and transcription factor co-binding shape the pioneering activity of Foxa2, Nucleic Acids Research (2019). DOI: 10.1093/nar/gkz627
Journal information: Nucleic Acids Research
Provided by Ludwig Maximilian University of Munich