Christie Sze, a fifth-year doctoral student in Feinberg’s Driskill Graduate Program in Life Sciences, was the first author of the study. Credit: Jim Prisching
The catalytic activity of an enzyme called Set1A—a protein that is essential to the viability of embryonic stem cells (ESCs)—is not required for ESC self-renewal, according to a Northwestern Medicine study published in the journal Genes & Development.
The findings provide important new insights into Set1A, a subunit of the COMPASS protein complex implicated in developmental disorders and some cancers.
"This surprised everyone in the lab," said Christie Sze, a fifth-year doctoral student in Feinberg's Driskill Graduate Program in Life Sciences, and the first author of the study. "It's an unexpected finding: even though this protein is absolutely important for embryonic stem cells, its main function is not."
The study also demonstrated that the catalytic function is necessary, however, during ESC differentiation, according to senior author Ali Shilatifard, PhD, the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics.
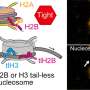
Set1A is one of six members of the COMPASS family of enzymes in humans. These proteins, which were first identified by Shilatifard in 2001, have since been shown to be central to gene expression through their role in implementing methylation at a histone location called H3K4.
Set1A appears to be particularly important; unlike other COMPASS members, embryonic stem cells without Set1A are unable to survive. Further, dysfunction of Set1A has previously been linked to developmental disorders, such as schizophrenia, and certain cancers.
Although it has been clear that the enzyme itself is critical in development, whether this was due to Set1A's main methylase activity was not understood.
In the current study, using a mouse embryonic stem cell model, Sze specifically targeted the SET domain of Set1A—the part of the protein that is responsible for its catalytic activity—with the gene-editing tool CRISPR.
The scientists discovered that after deleting Set1A's functional domain—rendering the enzyme unable to implement methylation—the embryonic stem cells still survived, with no effect on proliferation and self-renewal.
In the second part of the study, Sze investigated the mutant ESCs during differentiation, and demonstrated that Set1A's catalytic activity was indeed required for normal ESC differentiation.
"It's saying that in embryonic stem cells, H3K4 methylation by Set1A is not important, but in differentiation it is," said Shilatifard, also a professor of Pediatrics and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. "This brings up the question: what is special about histone H3K4 methylation during differentiation? And what is the function of the whole Set1A protein that is independent of its catalytic function?"
Going forward, the Shilatifard lab will investigate other aspects of Set1A, in order to uncover what about the enzyme itself is critical for embryonic stem cells, if it's not its catalytic function. The findings could help advance the understanding of cancer development.
"The COMPASS family is highly mutated in cancer. Although this study is from a developmental aspect, in order to understand cancer, you need to understand the normal functions of the protein," Sze said. "Our lab is continuing to learn more and more about each of these family members, and how they play a role in disease."
In an additional finding, data from the study also suggest that Set1A is responsible for activating bivalent genes—important for regulating key developmental processes—during differentiation. The lab had previously demonstrated that another COMPASS enzyme, called MLL2, is important for this activation in the self-renewing state; the current study suggests that there is a switch of responsibilities between the two enzymes during the different stages.
More information: Christie C. Sze et al. Histone H3K4 methylation-dependent and -independent functions of Set1A/COMPASS in embryonic stem cell self-renewal and differentiation, Genes & Development (2017). DOI: 10.1101/gad.303768.117
Journal information: Genes & Development
Provided by Northwestern University