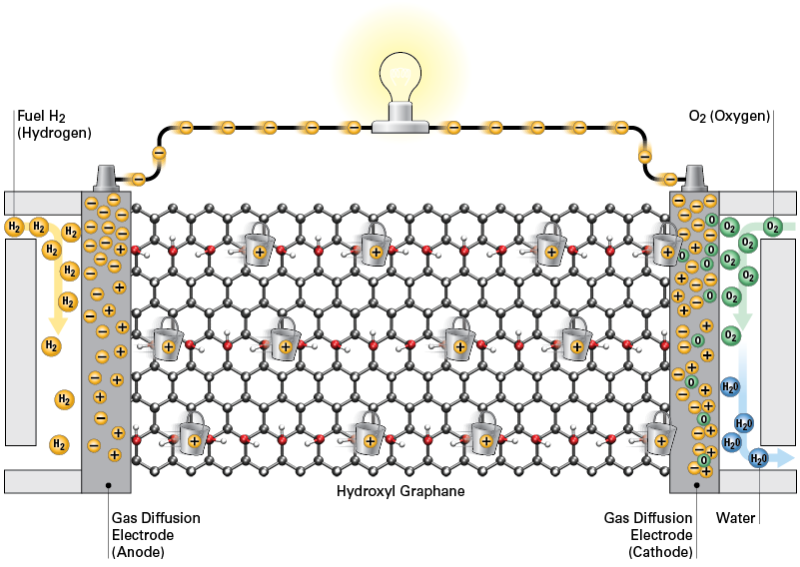
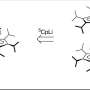
In computer simulations at Pitt, graphane provides a water-free "bucket brigade" to rapidly conduct protons across the membrane and electrons across the circuit. Credit: A. Bagusetty/University of Pittsburgh; Rick Henkel
Hydrogen powered fuel cell cars, developed by almost every major car manufacturer, are ideal zero-emissions vehicles because they produce only water as exhaust. However, their reliability is limited because the fuel cell relies upon a membrane that only functions in when enough water is present, limiting the vehicle's operating conditions.
Researchers at the University of Pittsburgh's Swanson School of Engineering have found that the unusual properties of graphane – a two-dimensional polymer of carbon and hydrogen – could form a type of anhydrous "bucket brigade" that transports protons without the need for water, potentially leading to the development of more efficient hydrogen fuel cells for vehicles and other energy systems.
The principal investigator is Karl Johnson, the William Kepler Whiteford Professor in the Swanson School's Department of Chemical & Petroleum Engineering, and graduate research assistant Abhishek Bagusetty is the lead author. Their work, "Facile Anhydrous Proton Transport on Hydroxyl Functionalized Graphane", was published this week in Physical Review Letters. Computational modeling techniques coupled with the high performance computational infrastructure at the University's Center for Research Computing enabled them to design this potentially groundbreaking material.
Hydrogen fuels cells are like a battery that can be recharged with hydrogen and oxygen. The hydrogen enters one side of the fuel cell, where it is broken down into protons (hydrogen ions) and electrons, while oxygen enters the other side and is ultimately chemically combined with the protons and electrons to produce water, releasing a great deal of energy.
At the heart of the fuel cell is a proton exchange membrane (PEM). These membranes mostly rely on water to aid in the conduction of protons across the membranes. Everything works well unless the temperature gets too high or the humidity drops, which depletes the membrane of water and stops the protons from migrating across the membrane. Dr. Johnson explains that for this reason, there is keen interest in developing new membrane materials that can operate at very low water levels–or even in the complete absence of water (anhydrously).
"PEMs in today's hydrogen fuel cells are made of a polymer called Nafion, which only conducts protons when it has the right amount of water on it," says Dr. Johnson. "Too little water, the membrane dries out and protons stop moving. Too much and the membrane "floods" and stops operating, similar to how you could flood a carbureted engine with too much gasoline," he added.
Dr. Johnson and his team focused on graphane because when functionalized with hydroxyl groups it creates a more stable, insulating membrane to conduct protons. "Our computational modeling showed that because of graphane's unique structure, it is well suited to rapidly conduct protons across the membrane and electrons across the circuit under anhydrous conditions," Dr. Johnson said. "This would enable hydrogen fuel cell cars to be a more practical alternative vehicle."
More information: Abhishek Bagusetty et al, Facile Anhydrous Proton Transport on Hydroxyl Functionalized Graphane, Physical Review Letters (2017). DOI: 10.1103/PhysRevLett.118.186101
Journal information: Physical Review Letters
Provided by University of Pittsburgh