Tailor-made membranes for the environment
The combustion of fossil energy carriers in coal and gas power plants produces waste gases that are harmful to the environment. Juelich researchers are working on methods to not only reduce such gases, but also utilize them. They are developing ceramic membranes with which pure hydrogen can be separated from carbon dioxide and water vapor. The hydrogen can then be used as a clean energy carrier, for example in fuel cells.
In technical systems, membranes can be used to separate gases - in a manner that is more efficient and cost-effective than with established methods. Membrane systems thus enable the separation of harmful greenhouse gases with comparatively low losses. At the same time, they also make it possible to obtain high-purity hydrogen for clean energy generation and storage, making ceramic membranes a key technology for transforming the energy sector (Energiewende).
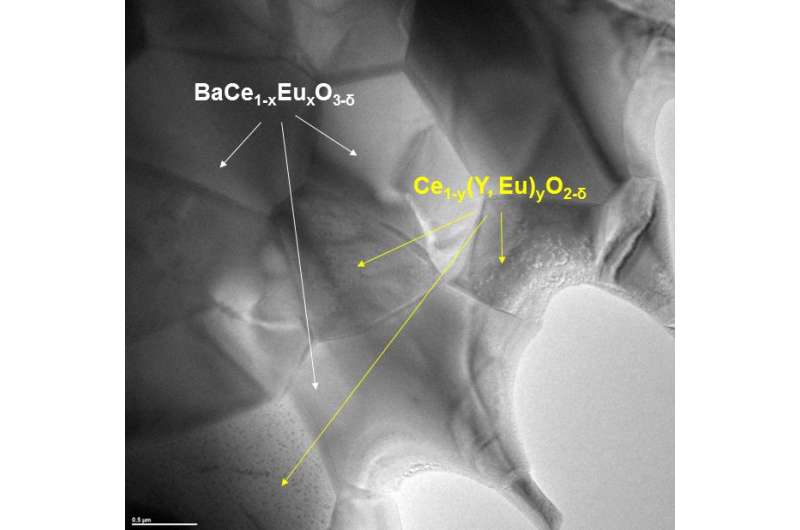
One option for separating hydrogen from gas mixtures is a two-phase membrane. "This consists of two ceramic materials. The individual grains are only a thousandth of a millimetre in size and exhibit both ionic and electronic conductivity," explains Dr. Mariya Ivanova from the Juelich Institute of Energy and Climate Research. The components of the hydrogen - protons and electrons - are thus transported individually through the membrane. On the other side, they combine to form high-purity hydrogen. This is made possible by tailor-made vacancies in the crystal lattice of the ceramics, which are occupied by protons. These protons, driven by pressure differences and temperature, are conducted through the material of the membrane. "They dock onto a hydrogen ion and jump in the direction of the lower pressure to the next hydrogen ion, from vacancy to vacancy, until they form elementary hydrogen again on the other side," says Mariya Ivanova. "The electrons are transported through the second component of the ceramic and ensure that charge equalization occurs."
However, the method still has a number of weak points. For example, high temperatures are needed for hydrogen separation, thus meaning it requires a lot of energy. In addition, the membranes investigated so far are not stable and become unusable in a carbonaceous environment. The hydrogen flow rate is also not yet high enough. Nevertheless, the researchers headed by Mariya Ivanova have made some significant progress: by inserting foreign atoms into the crystal lattice, their membrane is more stable and can be used at lower temperatures. However, the greatest achievement is the increased hydrogen flow. "It is nearly twice as high as in all other cases that have been documented to date," says a delighted Ivanova.
The Juelich membranes used for the measurements are only the size of a 10 cent coin and half a millimetre thick. "It is still too early to be thinking about an industrial application," explains Ivanova. "We will continue to conduct research, searching for a suitable material with a high flow rate and stability as well as low costs. The next step will be to increase component size to make it fit for industrial application." The researchers are initially aiming to achieve an area of ten by ten square centimetres.
More information: Mariya E. Ivanova et al, Hydrogen separation through tailored dual phase membranes with nominal composition BaCe0.8Eu0.2O3-δ:Ce0.8Y0.2O2-δ at intermediate temperatures, Scientific Reports (2016). DOI: 10.1038/srep34773
Journal information: Scientific Reports
Provided by Forschungszentrum Juelich


















