Credit: Higher Education Press Limited Company
Membrane separation technology has become one of the most effective methods for water treatment, owing to its advantages of high separation efficiency, convenient operation and low-energy consumption. The membranes prepared from graphene oxide (GO) have received extensive attention for their high water permeability which is attributable to rapid water transport in the atomically smooth sp2 regions (non-oxidized regions) of their membrane channels.
Reduced graphene oxide (RGO) membranes are theoretically more conducive to the rapid transport of water molecules in their channels compared with graphene oxide (GO) membranes, as they have fewer oxygen-containing functional groups and more non-oxidized regions.
However, experimental results from literature indicated that the RGO membranes generally show very low water permeabilities, even lower than 1.0 L/(m2·h·bar). The reduction process of GO to RGO can decrease the number of oxygen functional groups on RGO nanosheets, thus, RGO membranes exhibit weaker hydrophilicity and narrower interlayer spacing.
Despite rapid transport of water molecules in the non-oxidized regions of RGO membrane channels, their weakly hydrophilic and narrow membrane channels could hamper the entry of water molecules into the channels, resulting in lower water permeability. In addition, the reduction of oxygen functional groups on the RGO membrane surface would weaken the electrostatic interactions between the membrane and charged species, which could lower the rejection rates for these charged species.
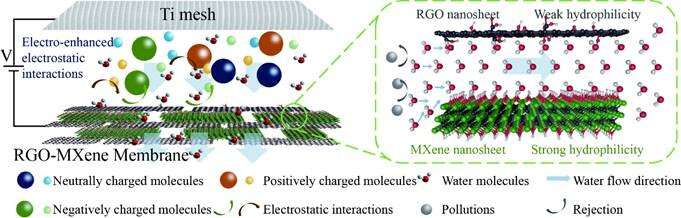
In order to solve the problem that the weak hydrophilicity of RGO membrane inhibits the water entry channel and leads to low water permeability, Prof. Xie Quan from Dalian University of Technology and his team members have constructed electroconductive MXene-intercalated RGO (RGO-MXene) membranes with wettability-regulated channels by intercalating MXene into RGO membranes.
This study entitled "Electroconductive RGO-MXene membranes with wettability regulated channels: improved water permeability and electro-enhanced rejection performance" is published online in Frontiers of Environmental Science & Engineering.
The research team found the RGO-MXene composite membrane exhibits high pure water permeance of 62.1 L/(m2·h·bar), approximately 16.8 times that of the RGO membrane (3.7 L/(m2·h·bar)). Wettability test results and molecular dynamics simulations suggest that the improved water permeance results from the enhanced wettability of RGO-MXene membrane and increased rate of water molecules entering the RGO-MXene channels.
Benefiting from good conductivity, the RGO-MXene membrane with electro-assistance exhibits significantly increased rejection rates for negatively charged dyes (from 56.0% at 0 V to 91.4% at 2.0 V for Orange G) without decreasing the permeate flux, which could be attributed to enhanced electrostatic repulsion under electro-assistance.
In this work, electroconductive RGO-MXene membranes are prepared by intercalating MXene into RGO membranes. The wettability of the membrane is regulated by tuning the mass percentage of MXene in the composite membrane. Wettability characteristics and molecular dynamics simulations reveal that membrane wettability and the rate of water molecules entering the membrane channels are important factors for improving water flux.
In addition, the RGO-MXene membrane exhibits improved rejection rates for charged dyes under electro-assistance. This work is expected to provide a new perspective for future research on the construction of novel membrane channel structures with high water transport and molecule rejection.
More information: Xiaoying Wang et al, Electroconductive RGO-MXene membranes with wettability-regulated channels: improved water permeability and electro-enhanced rejection performance, Frontiers of Environmental Science & Engineering (2022). DOI: 10.1007/s11783-023-1601-8
Provided by Higher Education Press