This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Membrane protein analogs could accelerate drug discovery
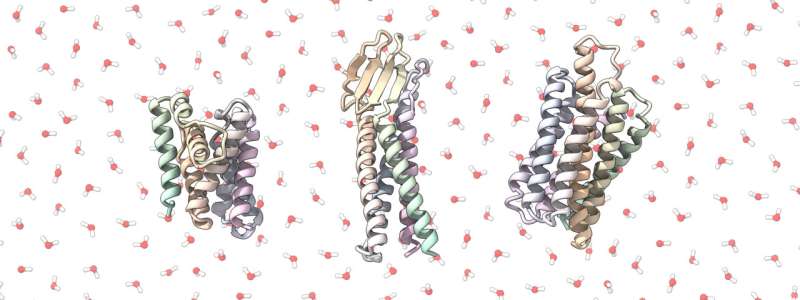
Many drug and antibody discovery pathways focus on intricately folded cell membrane proteins: when molecules of a drug candidate bind to these proteins, like a key going into a lock, they trigger chemical cascades that alter cellular behavior. But because these proteins are embedded in the lipid-containing outer layer of cells, they are tricky to access and insoluble in water-based solutions (hydrophobic), making them difficult to study.
"We wanted to get these proteins out of the cell membrane, so we redesigned them as hyperstable, soluble analogs, which look like membrane proteins but are much easier to work with," explains Casper Goverde, a Ph.D. student in the Laboratory of Protein Design and Immunoengineering (LPDI) in the School of Engineering.
In a nutshell, Goverde and a research team in the LPDI, led by Bruno Correia, used deep learning to design synthetic soluble versions of cell membrane proteins commonly used in pharmaceutical research. Whereas traditional screening methods rely on indirectly observing cellular reactions to drug and antibody candidates, or painstakingly extracting small quantities of membrane proteins from mammalian cells, the researchers' computational approach allows them to remove cells from the equation.
After designing a soluble protein analog using their deep learning pipeline, they can use bacteria to produce the modified protein in bulk. These proteins can then bind directly in solution with molecular candidates of interest.
"We estimate that producing a batch of soluble protein analogs using E. coli is around 10 times less expensive than using mammalian cells," adds Ph.D. student Nicolas Goldbach.
The team's research has recently been published in the journal Nature.
Flipping the script on protein design
In recent years, scientists have successfully harnessed artificial intelligence networks that use deep learning to design novel protein structures, for example by predicting them based on an input sequence of amino acid building blocks. But for this study, the researchers were interested in protein folds that already exist in nature; what they needed was a more accessible, soluble version of these proteins.
"We had the idea to invert this deep learning pipeline that predicts protein structure: if we input a structure, can it tell us the corresponding amino acid sequence?" explains Goverde.
To achieve this, the team used the structure prediction platform AlphaFold2 from Google DeepMind to produce amino acid sequences for soluble versions of several key cell membrane proteins, based on their 3D structure. Then, they used a second deep learning network, ProteinMPNN, to optimize those sequences for functional, soluble proteins.
The researchers were pleased to discover that their approach showed remarkable success and accuracy in producing soluble proteins that maintained parts of their native functionality, even when applied to highly complex folds that have so far eluded other design methods.
'The holy grail of biochemistry'
A particular triumph of the study was the pipeline's success in designing a soluble analog of a protein shape known as the G-protein coupled receptor (GPCR), which represents around 40% of human cell membrane proteins and is a major pharmaceutical target.
"We showed for the first time that we can redesign the GPCR shape as a stable soluble analog. This has been a long-standing problem in biochemistry, because if you can make it soluble, you can screen for novel drugs much faster and more easily," says LPDI scientist Martin Pacesa.
The researchers also see these results as a proof-of-concept for their pipeline's application to vaccine research, and even cancer therapeutics. For example, they designed a soluble analog of a protein type called a claudin, which plays a role in making tumors resistant to the immune system and chemotherapy.
In their experiments, the team's soluble claudin analog retained its biological properties, reinforcing the pipeline's promise for generating interesting targets for pharmaceutical development.
More information: Casper A. Goverde et al, Computational design of soluble and functional membrane protein analogues, Nature (2024). DOI: 10.1038/s41586-024-07601-y
Journal information: Nature
Provided by Ecole Polytechnique Federale de Lausanne