This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Refining turbulent flow to scale up iPS cell-based platelet manufacturing
In a recent effort to scale up the manufacturing of platelets using iPS cell-derived megakaryocytes, Professor Koji Eto (Department of Clinical Application) and his team of researchers, through computational simulation and biological testing, conceived new design changes for an impeller-based bioreactor for large-scale, high-quality platelet production. The study is published in Communications Engineering.
iPS cell-derived expandable immortalized megakaryocyte progenitor cell lines (imMKCLs) represent a renewable means to produce large amounts of platelets ex vivo for transfusion. Despite generating 100 billion (1011) competent iPS cell-derived platelets using a 10-L tank system previously by recreating turbulent flow with optimal turbulent energy and shear stress, true industrial-scale manufacturing is necessary for a consistent supply of transfusable platelets for patients with thrombocytopenia and other platelet disorders.
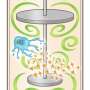
As such, the team began this study by developing a 50 L good manufacturing practices (GMP) grade, single-use United States Pharmacopeia standard (USP) class IV polyethylene tank and a new motor regulator for competent platelet production at even larger scales.
Through computation simulation of the turbulent energy and shear stress created in such 50 L tanks by computational fluidic dynamic (CFD) analysis, the researchers determined optimal motion speed, for the larger system to mimic conditions inside the smaller tanks examined previously. Notably, the larger tank consistently showed lower efficiencies than the smaller tanks tested (3 or 10 L) even though speed was optimized to generate similar turbulent energy and shear stress as an average value.
Furthermore, not only were platelets produced by the higher capacity 50 L tank lower in quantity, but they were also qualitatively poorer and showed ultrastructural abnormalities upon examination by transmission electron microscopy. Ultimately, they showed lower performance when the research team assessed their functions using in vitro assays or in vivo (hemostatic and circulatory kinetics) after transfusion into mice.
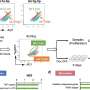
To gain biological insights into the failure to produce high-quality platelets after scaling up, the research team collected imMKCLs on days 3 and 5 of platelet production under different motion speeds and analyzed them by RNA sequencing. Principal component analysis revealed that the culturing conditions substantially altered imMKCL gene expression profiles.
Whereas imMKCLs under optimal conditions (corresponding to smaller tank cultures) upregulated genes related to angiogenesis, cell adhesion, cytoskeleton, hypoxia, platelet function, and TGF-β signaling, imMKCLs under excess speed, in contrast, upregulated genes associated with inflammation and damaged mitochondria function, consistent with the production of less healthy platelets.
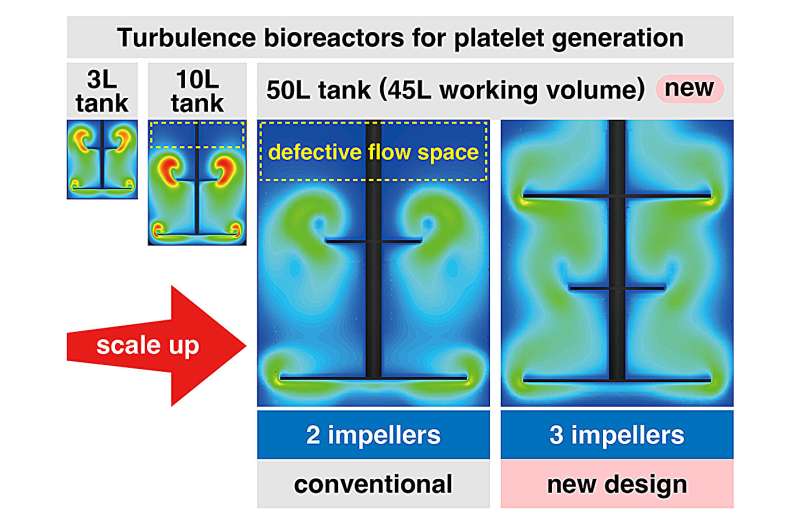
Finally, since it became clear that scaled-up platelet production conditions were not ideal, the research team returned to the drawing board and performed additional CFD simulations to determine whether turbulent flow became nonoptimal after scaling up. Notably, they discovered through this analysis that there is a substantial space with nonoptimized turbulent flow within the larger 50 L tank.
To minimize this undesired space (non-turbulence volume), the research team simulated a three-level impeller system and found that it should reduce the volume with defective turbulent flow. However, such a system is currently not commercially available, and hence, the researchers developed a new bioreactor system to ensure a more uniform distribution of cells and turbulent flow. A small 3 L system was constructed, which showed highly efficient production of high-quality platelets as expected.
Although additional work is needed to build a larger scale system and test the new bioreactor design, the research team expects the scaling-up process to go smoother next time because the new design does not share the same limitations.
More information: Haruki Okamoto et al, Defective flow space limits the scaling up of turbulence bioreactors for platelet generation, Communications Engineering (2024). DOI: 10.1038/s44172-024-00219-y
Provided by Kyoto University