December 11, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Genetically engineered cell therapies with mRNA lipid nanoparticles for transferrable platelets
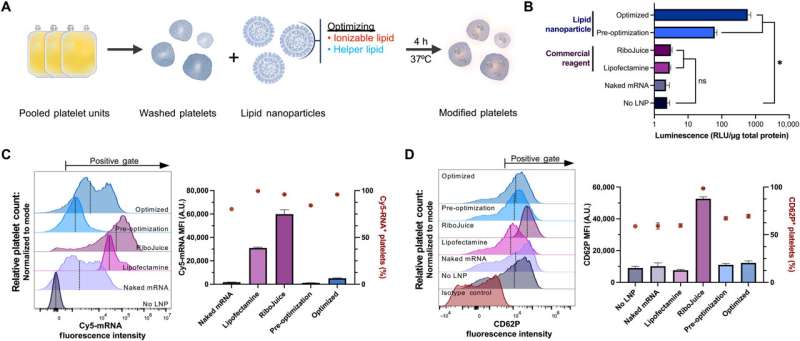
Platelet transfusions are essential in managing bleeding and hemostatic dysfunction, and can be expanded to be used as cell therapy for a variety of diseases. The efforts to create such cell therapies require that researchers modify donor platelets to express therapeutic proteins. However, at present, appropriate methods to genetically modify platelets collected from blood donors remain elusive.
In a new study published in Science Advances, Jerry Leung, and a team of scientists in nanomedicine, biochemistry, and molecular biology at the University of British Columbia, Canada, the Hokkaido University, Japan, and various institutions in the U.S. described an approach based on platelet-optimized lipid nanoparticles containing mRNA for exogenous protein expression in human and rat platelets.
When the team tested the library of mRNA-lipid nanoparticles, the resulting exogenous protein expression did not correlate with platelet activation. The transfected platelets retained hemostatic function and accumulated in areas of vascular damage after transfusion into rats with the capacity to expand the therapeutic potential of the platelets.
Platelets and hemostasis
Platelets are integral to hemostasis and are routinely transfused to restore hemostatic balance in patients. These platelets can be expanded beyond indications such as cell therapies to treat sepsis, inflammation and arthritis. Genetically modified platelets can create new cell therapies that express therapeutic proteins, which can be implemented to modify donor platelets. Existing methods of electroporation, viral vectors and commercial transfection have not been able to edit donor platelets and express exogenous proteins.
Indirect approaches can express exogenous proteins in platelets or platelet-like particles by targeting platelet precursor stem cells with lentiviral vectors. The donor-derived platelets must be functionally modified to create authentic platelet cell therapies.
Prior attempts to transfect platelets with lipid nanoparticles containing mRNA have shown the possibility of mRNA delivery into the platelets, while advances in lipid nanoparticle technology have improved its potential to reach a broader demographic.
In this work, Leung and colleagues reported mRNA lipid nanoparticles for their capacity to directly transfect donor platelets to express exogenous proteins. Such platelets can be modified with mRNA lipid nanoparticles to maintain their function and accumulate locally in wounds and regulate homeostasis after transfusion in coagulopathic rats.
Lipid nanoparticles facilitate exogenous protein expression in platelets
To identify the effective transfection methods for platelets, the team delivered mRNA encoding an enzyme NanoLuc luciferase (NanoLuc) using several transfection agents and measured their expression. While NanoLuc was undetected in platelets treated with free mRNA without a transfection agent or by using commercial mRNA delivery agents, the process allowed the uptake of large amounts of mRNA into the platelets.
Leung and team detected NanoLuc expression by using an mRNA-lipid nanoparticle formulation that resembled the small interfering RNA-lipid nanoparticle clinically proven to treat hereditary amyloidosis. The team compared the amount of platelet activation following mRNA lipid nanoparticle transfection to untreated platelets.
To identify the mRNA-lipid nanoparticle formulation most suited to transport platelets, they optimized three major components; ionizable lipids, linker lipids, and the polyethylene glycol lipid. They screened 10 ionizable and two permanently cationic lipids and measured their protein expression, mRNA uptake and activation to support protein synthesis.
Actions of the functionalized lipid nanoparticles in the lab
To examine how the combination of ionizable and helper lipids have synergistic effects to enhance protein expression while minimizing platelet activation, the team studied two FDA-approved ionizable lipids. Aside from their lipid composition, the mRNA elements played a significant role to promote efficient exogenous protein synthesis. The lipid nanoparticles containing helper lipids with a phosphocholine head, alongside lipids with branched or unsaturated tail groups were most suited for platelet transfection and to drive higher expression levels.
Of the RNA modifications tested in this work, Leung and colleagues noted unmodified uridine, or pseudouridine to facilitate higher expression levels of fluorescence. They then observed if the expression of fluorescence depended on the degree of platelet activation, or on the amount of RNA delivered, which they studied using a correlation matrix analysis.
While the fluorescence expression did not strongly correlate with surface platelet levels, or mRNA uptake, they noted a mild positive correlation between the amount of RNA delivered and platelet activation. Since NanoLuc expression did not strongly correlate with either surface platelet levels or mRNA uptake, the team tested the possibility of influencing its expression by activating platelets using agonists before and after mRNA lipid nanoparticle transfection.
Platelet treatment to model dilutional coagulopathy
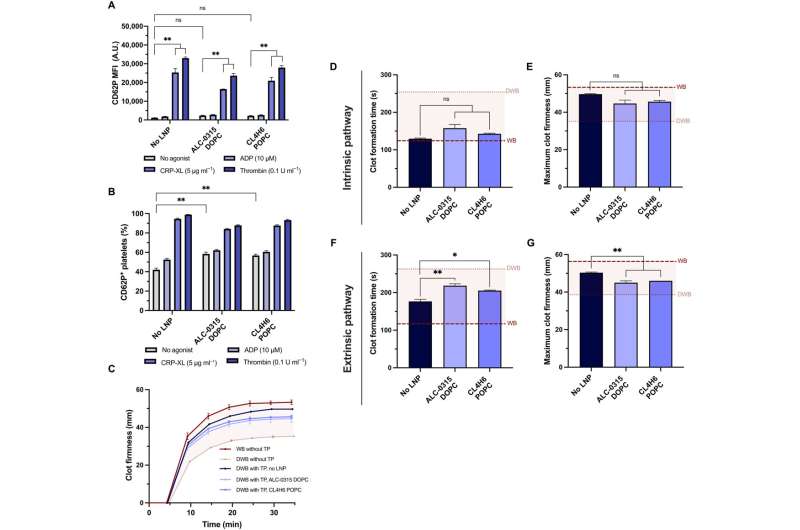
Platelets stimulated with adenosine diphosphate, for instance, cross-linked collagen-related peptide or thrombin prior to the mRNA-lipid nanoparticle treatment, had significantly less fluorescence expression. When Leung and the team stimulated platelets with agonists for more than two hours, they underwent substantial rearrangement of the transcriptome and proteome. The results showed that the translation of exogenous mRNA did not require platelet activation.
When the team treated platelets with mRNA lipid nanoparticles, they maintained hemostatic function in vitro and showed high sensitivity to their physical and chemical environments. The team investigated if the platelets could still be activated after mRNA-lipid nanoparticle transfection and measured their activation state and response to physiological agonists.
The team tested the capacity of transfected platelets to retain their potential to contribute to the firmness and rate of clot formation using a model of rotational thromboelastometry and an ex vivo model to test platelet activity in whole blood. The researchers modeled dilutional coagulopathy using diluted whole blood and prepared platelets in a transfusion package.
When they combined the transfusion package with diluted whole blood to model the disease as it occurs in a patient, they noted that the lipid nanoparticles did not affect platelet coagulopathy in vitro. Additionally, the team explored the expression of platelets transfected with mRNA lipid nanoparticles expressed with NanoLuc, circulated and localized into wound sites after transfusion into coagulopathic rodents.
Outlook
In this way, Jerry Leung and colleagues targeted the delivery of molecules and cell therapies into vasculature sites of interest by using the naturally competent platelets that can inherently perform this task. The team developed platelet-optimized lipid nanoparticles and mRNA for successful protein expression, while presenting platelet circular function and local accumulation at the vasculature site of interest.
It is possible to achieve the delivery of nucleic acids and exogenous translation using platelet-optimized mRNA-lipid nanoparticles to broaden and engineer platelets for a variety of clinical applications. Such donor platelets engineered with mRNA-lipid nanoparticles can treat acute bleeding disorders with broader applications in oncology. These platelets transfused with optimized mRNA-lipid nanoparticles are functionally transfusable and can accumulate at the vasculature site for effective platelet therapies to modulate hematological disorders.
More information: Jerry Leung et al, Genetically engineered transfusable platelets using mRNA lipid nanoparticles, Science Advances (2023). DOI: 10.1126/sciadv.adi0508
Journal information: Science Advances
© 2023 Science X Network