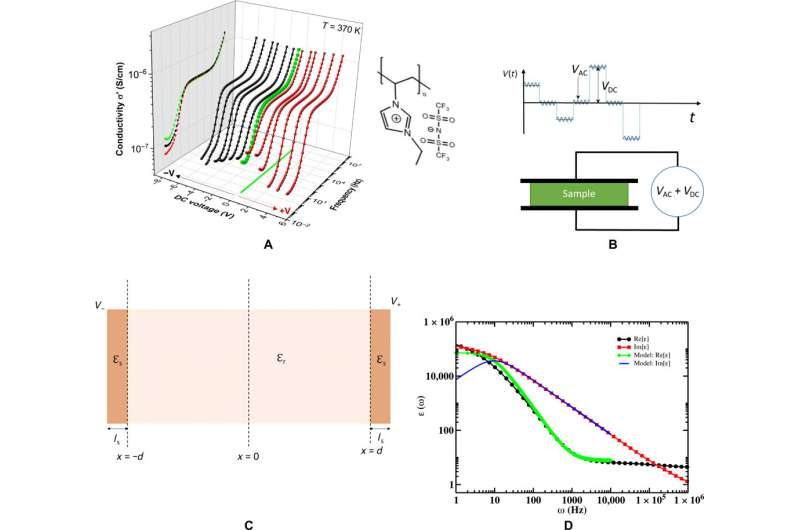
Details of the broadband dielectric spectroscopy (BDS) measurements, chemical structure of PolyIL studied in this work along with the electrode polarization model used for interpretation of experimental results. (A) BDS spectra plotted versus frequency and applied DC bias, along with the chemical structure of the PolyIL studied in this work. (B) Schematics of the applied voltage pulses involving DC and AC components as a function of time (t). (C) Schematic of the system studied using the Rayleighian approach (31) showing a film sandwiched between two planar parallel electrodes. The applied voltage at the right and the left electrode is V+ and V−, respectively. The material studied in this work, i.e., PolyIL and its counterions, has a relative permittivity of ϵr, and each electrode has an effective interfacial layer of the same thickness, ls, having a dielectric of ϵs < ϵr. (D) An example showing fittings of real and imaginary parts of BDS spectra using the electrode polarization model based on the Rayleighian approach. Credit: Science Advances, doi: 10.1126/sciadv.aba7952
Electrode-polymer interfaces can dictate properties of thin films including their capacitance, electric field, and charge transport, but scientists remain to fully comprehend their interfacial dynamics. In a new report on Science Advances, Rajiv Kumar, and a team of interdisciplinary scientists in the U.S. and Poland investigated electrified interfaces of an imidazolium-based polymerized ionic liquid (PolyIL) to understand the electric field induced transformations at the electrode-polymer interface. To achieve this, they used combinations of broadband dielectric spectroscopy (BDS), specular neutron reflectivity and molecular dynamics simulations. The capacitance depended on the applied voltage, which originated from the responses of an adsorbed polymer layer. The work will provide insight to features that affect the structure and properties of electrode-polymer interfaces to design next-generation energy storage and harvesting devices.
The electrical double layer (EDL) is a universal feature of electrified interfaces common to all ionic materials that spontaneously forms to store electrical energy in devices such as supercapacitors. Scientists aim to understand correlations between the structure and properties of EDLs to control device characteristics, including the capacitance of electrochemical storage devices, and charging and discharging rates of batteries. Research in the past two decades has focused on understanding the structural changes of EDLs in an applied electric field relative to charge storage properties. The results have indicated an interplay between electrostatics and crowding effects to be responsible for the anatomy of the EDLs in ionic liquids (ILs). However, relatively little is known about the capacitance of polymer electrolytes such as polymerized ionic liquids (PolyILs) that form promising solvent-free organic electrolytes for applications in batteries, solar cells, actuators and supercapacitors. The PolyILs also have tunable mechanical properties with high stability and are non-flammable.
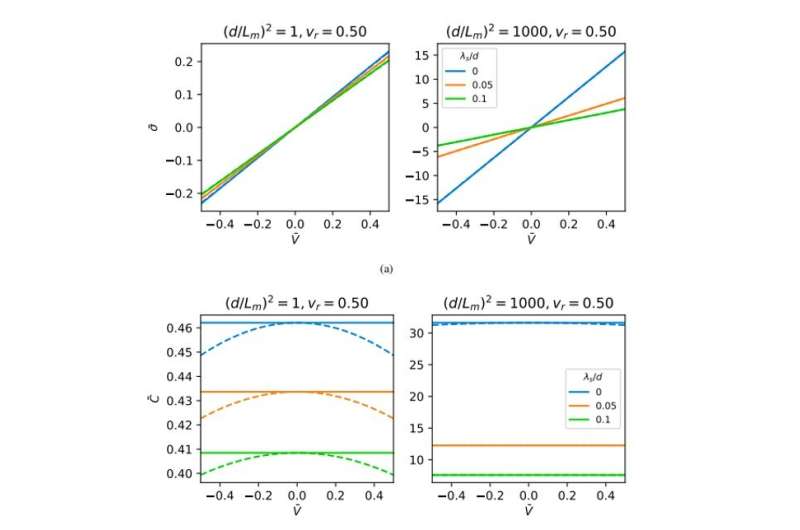
Surface charge (top panels) and differential capacitance (bottom panels) as a function of applied voltage, V¯. Solid lines: weakly inhomogeneous case, dashed lines: beyond the limit of weak inhomogeneity. Credit: Science Advances, doi: 10.1126/sciadv.aba7952
When a bias voltage is applied across an ionic liquid, scientists expect the cations to migrate toward the negative electrode and the anions to migrate toward the positively charged electrode to form an EDL (electrical double layer) on both electrodes. However, the structure of an EDL in PolyILs are currently not clear, although molecular dynamics simulations have offered insights with varying results for ionic liquids. Kumar et al. therefore investigated the electrode-polymer interfaces of a positively charged imidazolium-based PolyIL with bis(trifluoromethane) sulfonimide as counterions. They used combinations of broadband dielectric spectroscopy (BDS) and modelled the electrode polarization phenomena via the Rayleighian approach to obtain the capacitance of electrode-polymer interfaces and used the information to improve device storage properties.
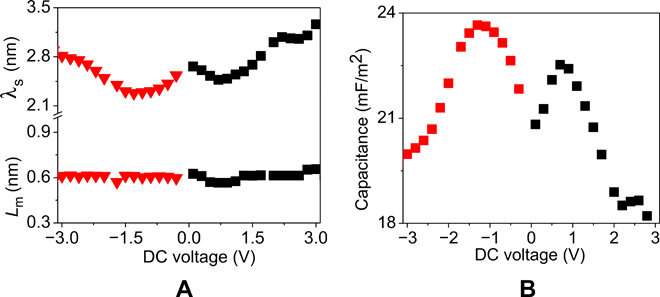
Voltage dependence of the capacitance at the steady state constructed from the apparent thickness of the low-dielectric layer and the length scale of mutual diffusion. (A) Apparent thickness of the low-dielectric layer (λs) and the length scale of mutual diffusion (Lm) obtained from the fitting of the BDS data for the PolyIL at 370 K with the Rayleighian-based electrode polarization model. Static dielectric constant of the film (ϵr) was also obtained by fitting spectra at zero DC voltage and kept constant as a function of the applied voltage. In particular, ϵr = 7.7 and d = 25 μm were used in generating these plots. (B) Capacitance calculated from equation 1 derived in the study using parameters in (A). Credit: Science Advances, doi: 10.1126/sciadv.aba7952
Broadband dielectric spectroscopy measurements
The team obtained representative broadband dielectric spectroscopy (BDS) measurements at different frequencies relative to the applied direct current (DC) voltages. Using the experimental protocol, they varied the applied voltage to obtain spectra containing three different regions. Typically researchers can determine the thicknesses of adsorbed and diffuse layers and capacitance via impedance spectroscopy and by fitting with equivalent electrical circuitry models. While useful, the physical interpretation of extracted quantities based on equivalent circuits can pose challenges. Kumar et al. therefore used an electrode polarization model to extract the capacitance from the BDS spectra based on a previously devised Rayleighian approach.
They then assumed that each electrode in the model would have a layer of low-dielectric material in contact with the polymeric film of uniform static dielectric constant. The team used the model to interpret the kinetics of charging in similar PolyILs based on spectroscopy measurements, which were in excellent agreement with pulse field gradient-nuclear magnetic resonance (PFG-NMR)-based measurements. Using the model, the scientists extracted the apparent thickness of the low-dielectric layer and the length scale of mutual diffusion for the PolyIL films. The thickness of the low dielectric layer had a nonmonotonic dependence on the applied voltage, which increased with the increasing DC voltage.
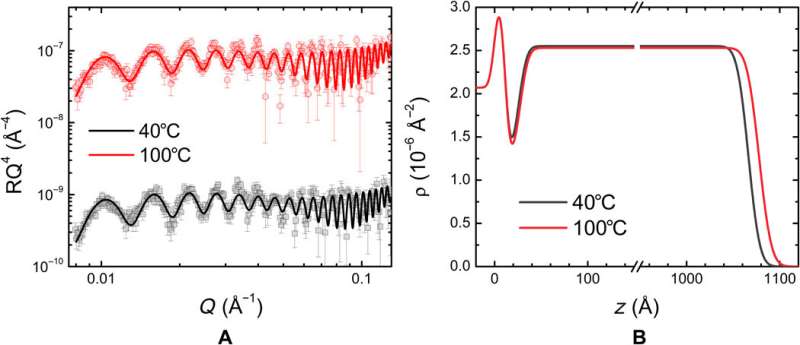
Results from neutron reflectometry showing the presence of an interfacial layer in films prepared by depositing the PolyIL at Si/SiO2 substrate. Neutron reflectivity (R) (A) and the associated SLD (ρ) models (B) of the PolyIL films. Black squares and red circles indicate measurements at 40° and 100°C, respectively. The solid lines in the reflectivity plots represent the best fits generated by the SLD profiles shown in (B). These profiles correspond to the PolyIL deposited on the Si/SiO2 substrate. Credit: Science Advances, doi: 10.1126/sciadv.aba7952
Capacitance-voltage curves
The team obtained camel-shaped capacitance-voltage curves with similarities to those predicted in atomistic molecular dynamics simulations of ionic liquids on rough surfaces. Both the silicon and metallic electrode surfaces used in the study had pre-adsorbed layers whose voltage dependence dictated the capacitance. Changes in the applied voltage therefore transformed the adsorbed layer to determine the capacitance-voltage relation, highlighting the importance of the quality and chemistry of the pre-adsorbed layer to design efficient energy storage devices. The team used a minimal model with simplifying assumptions and numerical equations derived in the study to construct the capacitance-voltage relations from BDS measurements.
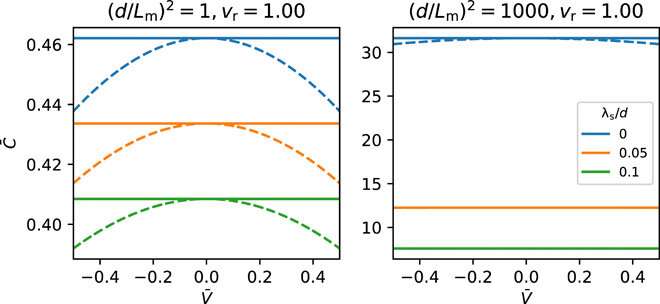
Results obtained for capacitance-voltage relations by numerically solving the underlying equations of the electrode polarization model in a steady state. Here, vr is the ratio of molar volume of a counterion to that of a monomer, C¯¯¯ is the capacitance (in units of ϵoϵr/d) and V¯¯¯=eV/kBT, V being the applied voltage. Solid lines are obtained by solving an approximate set of equations enforcing weak inhomogeneity, and dashed lines represent numerical solutions beyond the limit of weak inhomogeneity. Credit: Science Advances, doi: 10.1126/sciadv.aba7952
In this way, Rajiv Kumar and colleagues studied electrode PolyIL (polymerized ionic liquid) surfaces with broadband dielectric spectroscopy measurements, neutron reflectometry and modelling based approaches. They noted the presence of a pre-adsorbed layer at the electrode, which dictated the measured impedance and capacitance of the electrode-PolyIL interfaces. They expect the pre-adsorbed layer of the electrode polarization model to be present in most other films containing similarly charged PolyILs and substantially contribute to the capacitance. The scientists expect the phenomenon to improve energy storage and harvesting device applications.
More information: Rajeev Kumar et al. Capacitance of thin films containing polymerized ionic liquids, Science Advances (2020). DOI: 10.1126/sciadv.aba7952
Maxim V. Fedorov et al. Ionic Liquids at Electrified Interfaces, Chemical Reviews (2014). DOI: 10.1021/cr400374x
Matthew A. Gebbie et al. Long-range electrostatic screening in ionic liquids, Proceedings of the National Academy of Sciences (2015). DOI: 10.1073/pnas.1508366112
Journal information: Science Advances , Chemical Reviews , Proceedings of the National Academy of Sciences
© 2020 Science X Network