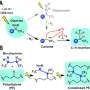
Molecular formulae of phosphors and polymer matrices. The molecular formulae of TPEDB, PVA with different alcoholysis degree (PVA50, PVA67, PVA100), and controlled polymers (PDDA, PSS, and PVDF). Credit: Science Advances, doi: 10.1126/sciadv.aaz6107
Polymer-based room-temperature phosphorescence (RTP) materials can be efficiently developed by covalently embedding phosphors into the polymer matrix. The process is still, however, highly challenging on a large-scale due to inefficient binding engineering and time-consuming covalent reactions. In a new report on Science Advances, Rui Tian, and a team of research scientists at the State Key Laboratory of Chemical Resource Engineering in China, proposed a scalable preparation approach for RTP materials. They used the B-O click reaction between boronic acid-modified phosphors and the polyhydroxy polymer matrix. The molecular dynamics simulations showed effective immobilization of phosphors to result in suppressed nonradiative transitions and activated RTP emission. The team completed these B-O click reactions within 20 seconds in ambient environments and the strategy introduced facile click chemistry to simplify the construction of polymer-based RTP polymeric materials. The successful outcomes of this study will allow large-scale production of RTP materials industrially.
Polymer-based room-temperature phosphorescence (RTP) materials have received increased attention in the past few decades in the field of flexible organic electronics due to multiple advantages including good flexibility, stretchability and low cost. Researchers have also observed great advances in polymer-based RTP materials synthesis in the past. Two main categories of the materials synthesis include non-doped polymer materials with phosphor in the backbone of the polymer itself and a second category of phosphors embedded in a polymer matrix to form doped RTP polymers. The doped materials could construct efficient RTP polymeric materials as a result of the polymer matrix that suppressed non-radiative transitions of phosphors to activate RTP generation. Existing doped RTP materials are implemented via noncovalent interactions (i.e. electrostatic interactions or van der Waals forces) between phosphors and polymer matrices, although such interactions formed nondirectional weak links that resulted in phase separation. Covalent crosslinking might overcome such deficiencies by forming strong C-O-C interactions.
Engineering room-temperature phosphorescence (RTP) materials with click chemistry
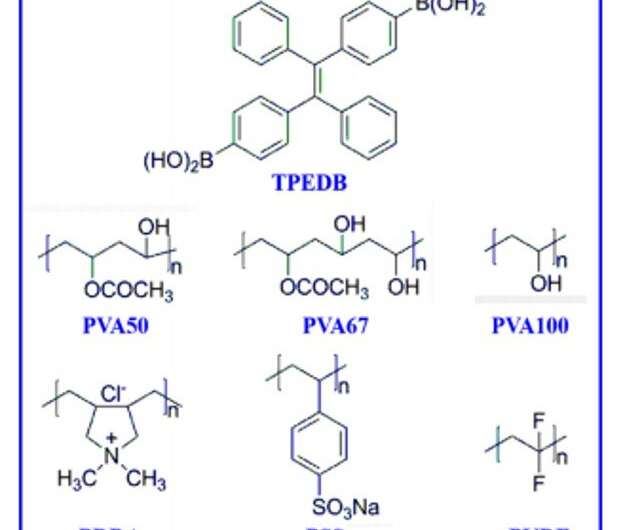
Schematic representation for the polymer-based room-temperature phosphorescence (RTP). Facile and large-scale approach of RTP through B─O click reaction between phosphors with boronic acid and polymer with hydroxyl groups. Credit: Science Advances, doi: 10.1126/sciadv.aaz6107
In this work, Tian et al. introduced a flexible and catalyst-free click reaction to synthesize covalently linked phosphor-polymer RTP materials. They constructed the efficient material through strong B-O covalent bonds between tetraphenylethylene-diboronic acid (abbreviated TPEDB) molecule and polyvinyl alcohol (PVA) matrix in 20 seconds under ambient environmental conditions. Based on the energetically favorable click reaction, the research team modulated the number of B-O covalent bonds via click tailoring to contribute to strong RTP intensity and a long life-time of up to 768.6 milliseconds to form the TPEDB-PVA polymeric material. Then using ab initio molecular dynamics (AIMD) simulations, Tian et al. credited the properties of efficient phosphorescence to suppressed molecular rotation and restricted non-radiative transition of TPEDB. The strategy provides a large-scale platform to manufacture and industrialize efficient polymer-based RTP materials for practical applications.
The fluorescence and pH results showed a covalent B-O click reaction, followed by the formation of a flexible RTP polymeric material. The team studied the morphology of the resulting polymer with scanning electron microscopy (SEM) and atomic force microscopy (AFM). They obtained a uniform and continuous surface for the material with a thickness of 27 µm and conducted elemental analyses with energy-dispersive X-ray spectroscopy (EDX) to map and detect a homogenous dispersion of boron, oxygen and carbon elements, as expected. The results showed a good combination between phosphors and the polymer matrices to form the TPEDB-PVA polymer.
Characterizing the material architecture and regulating the covalent bonds
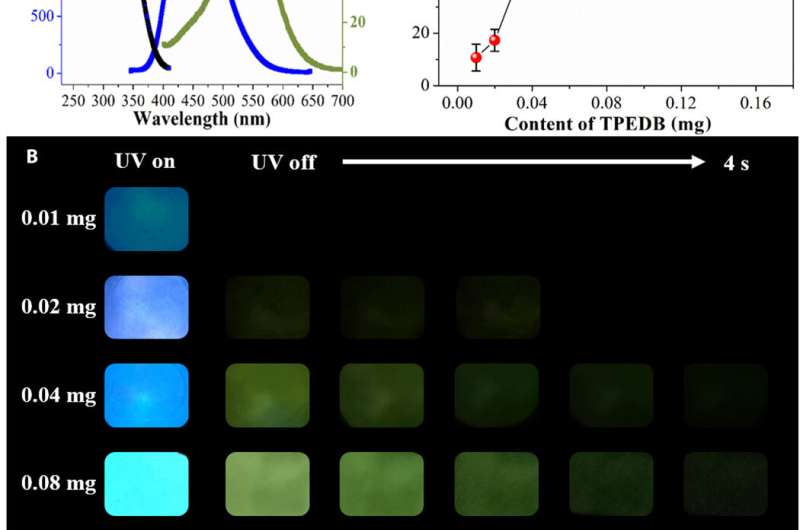
Luminescent behaviors of TPEDB-PVA polymeric materials. (A) Fluorescent excitation (black), emission (blue), and RTP emission (green) spectra of TPEDB-PVA polymeric material. (B) Photographs of TPEDB-PVA polymeric material under 365-nm ultraviolet (UV) irradiation and at different time intervals after removal of UV irradiation. Photo credit: Rui Tian (first author), Beijing University of Chemical Technology. (C) RTP intensities of TPEDB-PVA polymeric materials with different contents of TPEDB. Credit: Science Advances, doi: 10.1126/sciadv.aaz6107
Tian et al. recorded the luminescent spectra of the TPEDB-PVA materials to obtain fluorescent and green spectra for persistent lifetimes of 4.5 nanoseconds and 768.6 milliseconds, respectively. To study the origin of room temperature phosphorescence (RTP) of the materials, the team drop-casted TPEDB, PVA and TPEDB-X% PVA on quartz glass. The pristine TPEDB material showed weak RTP emission and the addition of PVA to the mix promoted RTP performances to indicate the role of PVA as a matrix to activate the phosphorescence of TPEDB. The scientists achieved the strongest RTP intensity for polymeric materials when the PVA reached 60 mg and determined the optimum content of TPEDB to be 0.08 mg in the combined TPEDB-PVA polymer. Further experiments investigated the superiority of the covalent bonds to show the necessity of both hydroxyl groups and boronic acid groups in the setup to form a stable covalent link for efficient RTP materials.
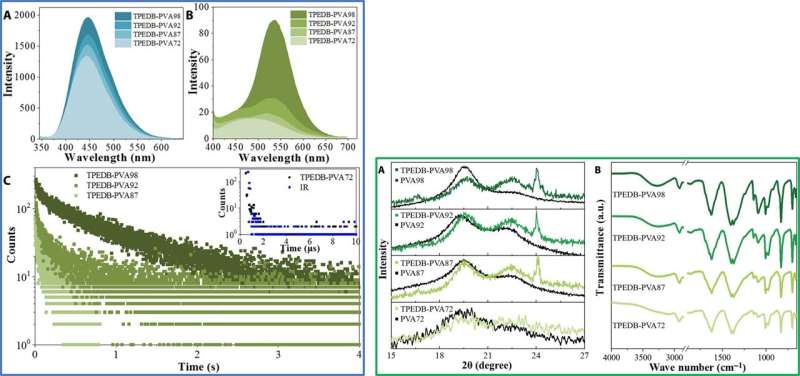
Since covalent cross-linkage between TPEDB and PVA were important for phosphorescence, the team applied different degrees of alcoholysis (or hydrolysis) to regulate covalent bonds and verify the speculation. They noted increasing phosphorescence and fluorescent performances with increasing alcoholysis degree of PVA. The team conducted X-ray diffraction (XRD) measurements and verified the interactions between the two constituents (TPEDB and PVA) under varied degrees of alcoholysis, followed by Fourier transform infrared (FTIR) measurements to observe the expected characteristic peak corresponding to the B-O bond in the polymers. The increased quantity of hydroxyl groups in PVA provided linking groups for both covalent and hydrogen bonds to form in the system, creating a favorable environment to confine the phosphors and activate their phosphorescence.
Understanding the mechanisms underlying the polymeric materials
LEFT: Luminescent performances of TPEDB-PVA polymeric materials with varied alcoholysis degree of PVA. (A) Fluorescent emission, (B) phosphorescent emission spectra, and (C) RTP lifetime of TPEDB-PVA polymeric materials (TPEDB of 0.08 mg) with alcoholysis degree of PVA ranged from 87%, 92%, to 98%, and the inset shows the radiative curve of TPEDB-PVA72 polymeric material (black) and the instrumental reference (blue). RIGHT: Structural studies for TPEDB-PVA polymeric materials. (A) XRD patterns and (B) FTIR spectra for TPEDB-PVA polymeric material (TPEDB of 0.08 mg) with alcoholysis degree of PVA ranging from 72%, 87%, 92%, to 98%. a.u., arbitrary units. Credit: Science Advances, doi: 10.1126/sciadv.aaz6107
To understand the B-O click reaction, the origin of phosphorescence and its enhancement through covalent localization of phosphor, the scientists performed density functional theory calculations. They computed the Gibbs free energy change of the click reaction (TPEDB + PVA —-> TPEDB-PVA + H2O) to be -1.017 eV, which indicated the energetic favorability of the reaction with an ultrafast reaction rate. They calculated the energy levels of the ground state, first singlet excited state and first triplet excited state for TPEDB and PVA, and the results showed the origin of phosphorescence from TPEDB, while PVA formed a non-emissive polymer matrix to stabilize the molecules. Tian et al. also performed AIMD (ab initio molecular dynamics) simulations to manipulate the structure, composition and orientation of TPEDB-polymer materials and understand their impact on phosphorescence.
Applications of TPEDB-PVA polymeric materials
The team then studied potential applications of the TPEDB-PVA polymer materials, their solubility and stability. The polymers dissolved completely in two minutes at 60 degrees C due to constituent hydroxyl groups after the B-O click reaction, while the fluorescence of dissolved materials weakened. They studied the photostability of the materials under UV irradiation due to the protection offered by the PVA matrix to the TPEDB. Based on the solution-processing ability and decent photostability, the team credited the polymer to be a potential candidate to construct optoelectronic polymer materials.
They prepared scalable polymeric materials in petri dishes in the lab with varying radii and conducted data-encryption on the polymers by encoding numbers, which appeared as intense cyan fluorescence after UV excitation. They achieved an encryption method on the TPEDB-PVA polymer after varied degrees of alcoholysis and manipulated its composition to create an anti-counterfeiting security link. As proof-of-concept they patterned the numbers "1 2 3" on the PVA substrate to observe them under UV irradiation, creating a powerful anti-counterfeiting and digital encoding tool via a facile click reaction.
Practicality of TPEDB-PVA RTP polymeric materials. (A) Solubility in water (the inset shows the photos captured under UV irradiation) [photo credit: Rui Tian (first author), Beijing University of Chemical Technology], (B) photostability under UV irradiation, (C) photographs of scalable preparation of TPEDB-PVA polymeric materials (radii of 0.5, 1.0, and 2.5 cm) [photo credit: Qi Xu (co-author), Beijing University of Chemical Technology], (D) lifetime-dependent data encryption, and (E) digital coding written by TPEDB ink on PVA under and after UV irradiation. Credit: Science Advances, doi: 10.1126/sciadv.aaz6107
In this way, Rui Tian and colleagues presented an efficient polymer-based RTP material using a one-step B-O click chemistry strategy. They regulated the RTP performance by the number of B-O covalent bonds. The simple, highly efficient and scalable preparation technique will open new possibilities for innovative engineering methods to construct polymeric RTP materials. The successfully developed RTP material construct will have many applications in data security and as light-emitting devices with potential to expand the strategy across diverse RTP materials.
More information: R. Tian et al. Large-scale preparation for efficient polymer-based room-temperature phosphorescence via click chemistry, Science Advances (2020). DOI: 10.1126/sciadv.aaz6107
undefined Kenry et al. Enhancing the performance of pure organic room-temperature phosphorescent luminophores, Nature Communications (2019). DOI: 10.1038/s41467-019-10033-2
Yu Xiong et al. Designing Efficient and Ultralong Pure Organic Room‐Temperature Phosphorescent Materials by Structural Isomerism, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201800834
Journal information: Science Advances , Nature Communications , Angewandte Chemie International Edition
© 2020 Science X Network
![Practicality of TPEDB-PVA RTP polymeric materials. (A) Solubility in water (the inset shows the photos captured under UV irradiation) [photo credit: Rui Tian (first author), Beijing University of Chemical Technology], (B) photostability under UV irradiation, (C) photographs of scalable preparation of TPEDB-PVA polymeric materials (radii of 0.5, 1.0, and 2.5 cm) [photo credit: Qi Xu (co-author), Beijing University of Chemical Technology], (D) lifetime-dependent data encryption, and (E) digital coding written by TPEDB ink on PVA under and after UV irradiation. Credit: Science Advances, doi: 10.1126/sciadv.aaz6107 Large-scale preparation of polymer-based room-temperature phosphorescence via click chemistry](https://scx1.b-cdn.net/csz/news/800a/2020/4-largescalepr.jpg)