Researchers are taking snapshots of chemical reactions in a trillionth of a second in the hope of developing the next generation of antibiotics and anti-viral drugs.
Using state-of-the-art laser technology, scientists at Cardiff University and the Rosalind Franklin Institute are creating 'freeze frame movies' of chemical reactions, with a starring role for a specific enzyme that could be used to make new drugs that are active against viruses, such as COVID-19.
The technology will enable the team to observe the chemistry that takes place within an enzyme over very short periods of time, which will allow scientists to gain an understanding of how protein structure enables the chemical reaction to take place.
This information will be essential for efforts by the team to re-engineer the enzyme so that it can be used to produce compounds with anti-viral properties in a quick and efficient way.
To achieve this, they are taking advantage of the powerful capabilities of an X-ray free electron laser (XFEL) located in Hamburg, Germany.
The XFEL can be used to obtain pictures of enzyme reactions in crystals by firing X-ray pulses lasting a femtosecond—one quadrillionth of a second. The bonds between individual atoms take around 10 femtoseconds to form during chemical reactions, meaning that the XFEL should be able to take snapshots of structures as they take shape within the enzyme.
Making this a reality, however, is a technically demanding feat that has not been reported for the class of enzymes being studied by the team.
The team will be paying specific attention to an enzyme that is present in Streptomyces, bacteria that commonly live in soil and decaying vegetation and which are responsible for the production of over two-thirds of the clinically useful antibiotics found in nature.
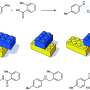
This enzyme facilitates the construction of a bond between two carbon atoms, a reaction described by the researchers as "the chemistry of life at its most basic".
Understanding this carbon-carbon bond forming reaction is important because it is found in C-nucleosides—a particular class of molecules that are extremely promising candidates for future anti-viral drugs. One example of a C-nucleoside is remdesivir, developed by Gilead Sciences, Inc., which is currently being trialled around the world as a potential treatment for COVID-19.
"We're essentially creating a freeze frame movie of chemistry in action," said project co-lead investigator Professor Nigel Richards from Cardiff University's School of Chemistry. "Chemical bonds form and break in very short periods of time, far too fast to be seen using other techniques. The new XFEL technology offers a solution to this problem for enzyme catalysed chemical reactions."
"This state-of-the-art technology will enable us to study biochemically important reactions like we've never been able to before, opening up a range of new possibilities for drug discovery and development.
"Our ground-breaking experiments will likely change how we think about chemical reactions that take place inside enzymes—a grand challenge of chemistry, biochemistry and biology. This will in turn allow us to engineer libraries of similar enzymes that can be used to make potential antibiotic and antiviral compounds, facilitating the drug discovery process."
"C-nucleosides are molecules of the future in drug discovery," continued Professor Richards. "These compounds are already widely used in nature to kill bacteria and viruses."
"Being able to use an enzyme to make the key carbon-carbon bond of C-nucleosides in the laboratory will allow us to build a variety of new compounds that can be evaluated as potential drugs," he said.
Provided by Cardiff University