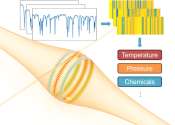
Optical barcodes expand range of high-resolution sensor
The same geometric quirk that lets visitors murmur messages around the circular dome of the whispering gallery at St. Paul's Cathedral in London or across St. Louis Union Station's whispering arch also enables the construction ...