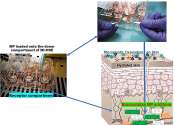
Toxic fireproof chemicals can be absorbed through touch, 3D-printed skin model shows
Cancer-causing flame retardants found in everyday things like plastics, furniture, fabrics and electronics can be sucked up by the skin and absorbed into the bloodstream in 24 hours, scientists have found.