A map of reactions helps control molecular properties
Chemical reactions are used to produce all kinds of important molecules such as drugs, agrochemicals and materials.
To create a drug with specific properties—polar enough to dissolve in the aqueous environment of the stomach and blood, but greasy enough to pass through cells or cross the blood-brain barrier—chemists usually swap out the starting materials.
Now, a University of Michigan study has shown that it's possible to alter the properties of molecules by modifying reaction conditions instead, potentially streamlining the way drugs and other useful compounds are discovered. The work is published in Nature.
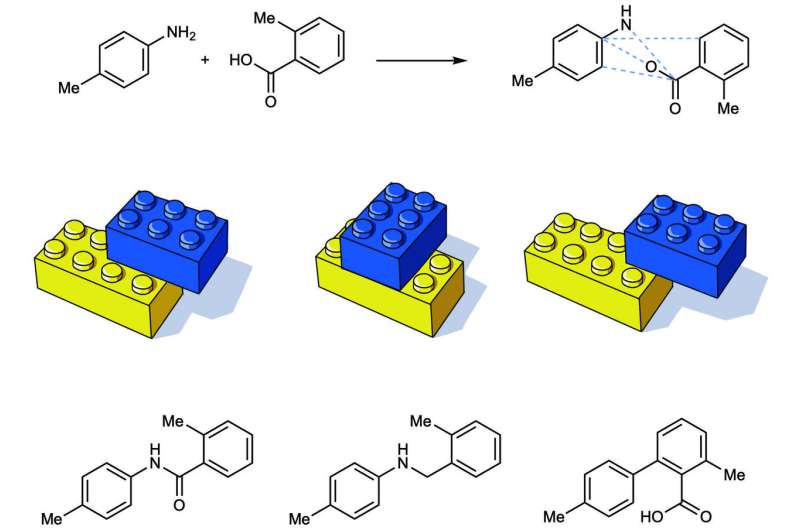
"Picture a box of Lego bricks. Grab any two and click them together. Maybe you get a red one and a blue one, or a black one and a yellow one. But if you only had two Lego bricks, you could still click them together in lots of ways to get different shapes. Picture clicking two Lego blocks together using two divots, four divots, or one divot. You get different shapes," said lead author Tim Cernak, U-M assistant professor of chemistry and medicinal chemistry.
Cernak's team is trying to do the same with molecules. Typically, chemists search for atoms with specific properties in order to build molecules with the desired outcomes. Instead, the U-M team suggests using different reaction conditions to make molecules with different shapes and different properties.
"By mapping out all of the possible reactions, we've uncovered that there is a completely new axis to consider when changing the properties of a molecule," Cernak said.
Twenty-five percent of reactions used in drug discovery bring together an amine and a carboxylic acid to form an amide bond. These bonds are formed by coupling the basic amine compound with a carboxylic acid. Now, the U-M team has shown that tweaking the way an amine and a carboxylic acid are connected can result in hundreds of other ways to click together the two starting materials, each producing a new molecule with diverse properties.
This means that by varying the catalyst and reagent that snap the amine and carboxylic acid together, chemists can engineer the properties the compound will display. Cernak's lab shows this method can produce molecules that are acidic, basic, lipophilic or hydrophilic, all initiating from the same two starting materials but using different reaction conditions.
"If we're talking about pharmaceuticals, these changes in properties could be the difference between a molecule that distributes to the brain and a molecule that goes to the liver," Cernak said. "This is exactly what drug hunters do: they change the atoms just a little bit to modulate the properties of molecules. That's always done by going to the Lego box and grabbing different pieces of Lego.
"What if you could do all of that with just two pieces of Lego simply by using different catalysts to snap them together in different ways? It adds a new dimension to chemical space exploration."
For example, if you decarboxylate the carboxylic acid instead of forming an amide bond, you form a carbon-nitrogen bond. If you remove the amine, you form a carbon-oxygen bond. If you simultaneously remove the amine and decarboxylate the carboxylic acid, you can make a carbon-carbon bond.
"We show that you can access carbon-nitrogen, carbon-oxygen and carbon-carbon bonds from the same two starting materials just by varying reaction conditions," Cernak said. "The discovery that keeps me awake at night is that there are hundreds of other reactions that we haven't yet realized. There is so much opportunity in these hundreds of new reactions that are just waiting to be invented."
The study also provides a computational framework to invent new reactions using a rapid reaction screening technique developed by Cernak.
"We are developing an interface of chemical synthesis and data science," he said.
More information: Babak Mahjour et al. A map of the amine–carboxylic acid coupling system, Nature (2020). DOI: 10.1038/s41586-020-2142-y
Journal information: Nature
Provided by University of Michigan