Light-induced modification of a carboxylic acid with an aminocyclopropenone
Researchers at Kanazawa University report in the Journal of Organic Chemistry that carboxylic acids, functional groups contained in biomolecules, drugs and materials, can be readily modified by light-induced organic reactions using an aminocyclopropenone. This discovery opens up new pathways for carboxylic acid modification with potential applications including determination of drug target proteins, elucidation of protein function, and synthesis of functionalized polymer materials.
A phototriggered reaction is a chemical reaction that is induced by irradiation with light. This technique is useful for biochemical studies aiming to gain better insight into the structure and function of proteins, by labeling them through phototriggered reactions. Another use of the reaction method is the modification of polymers. Now, a team of researchers from Kanazawa University has developed a phototriggered modification reaction of a carboxylic acid using an aminocyclopropenone.
The researchers have previously reported on the phototriggered dehydration condensation of a carboxylic acid and an amine using an aminocyclopropenone. (A carboxylic acid has a COOH group, with 'C' denoting carbon, 'O' oxygen and 'H' hydrogen. An amine is an organic molecule with a nitrogen atom bound to three organic substituents. An aminocyclopropenone is an organic compound with a three-membered carbon ring substituted with oxygen and nitrogen.) Irradiation with weak light cause the aminocyclopropenone to undergo decarbonylation to produce a highly reactive ynamine (A ynamine is an organic compound which contains a C≡C triple bond substituted with nitrogen). The resulting ynamine works as a dehydration agent to connect the carboxylic acid and the amine.
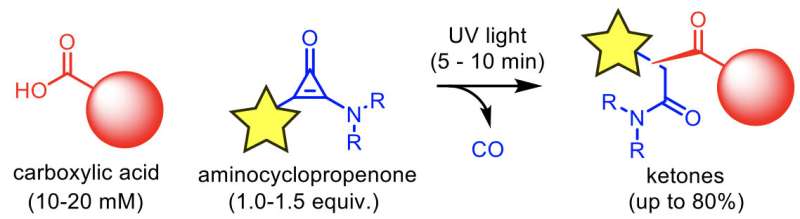
When the research team investigated the detailed conditions of the phototriggered dehydration condensation, they found that another reaction occurred under intense light conditions. In this reaction, an isomeric mixture of three ketones was produced (A ketone has a C=O double bond, with two substituents attached to the carbon atom.). The ketones have substituents derived from the carboxylic acid and aminocyclopropenone. In other words, the carboxylic acid is modified with substituents on the aminocyclopropenone.
The researchers hypothesized that the reaction proceeds via the following three steps. (i) An aminocyclopropenone is photolyzed to produce an ynamine. (ii) A carboxylic acid reacts with the ynamine to produce an acyloxyenamine. (An acyloxyenamine is a 1:1 adduct of a carboxylic acid and an ynamine.) (iii) The acyloxyenamine is photolyzed to produce an isomeric mixture of ketones. The researchers isolated the acyloxyenamine intermediate and investigated its optical properties and chemical reactivity. The results of the investigation strongly supported the hypothetical mechanism.
Various primary and secondary carboxylic acids including amino acid derivatives were modified with the phototriggered reaction. Under optimized conditions, the ketones were obtained in up to 80% combined yield. Using a terminal alkyne substituted aminocyclopropenone, the alkyne group was introduced into a carboxylic acid (alkyne features a C≡C triple bond). Because various functional groups can be selectively introduced to the alkyne scaffold by alkyne azide click chemistry, a broad range of functional groups can be introduced into a carboxylic acid by combination of the phototriggered alkyne introduction and the alkyne azide click reaction.?Application of this phototriggered reaction for modifying proteins and materials is now under investigation in the authors' group.
More information: Kenji Mishiro et al, Phototriggered Ketone Formation from an Aminocyclopropenone and a Carboxylic Acid, The Journal of Organic Chemistry (2018). DOI: 10.1021/acs.joc.8b02250
Provided by Kanazawa University