Light and copper catalysis improves amine synthesis
EPFL chemists have developed a novel and efficient method to make amines, which are among the most important structural compounds in pharmaceuticals and organic materials. The study is published in Nature Catalysis.
Amines are molecules that contain a basic nitrogen atom. They are derived from ammonia, where one or its hydrogen atoms has been replaced with a carbon group, most commonly an aryl (ring-structured hydrocarbons) or alkyl group.
Amines are used widely in bioactive molecules, drugs, and various organic materials, and preparing them is one of the most important tasks for synthetic chemists in both academia and industry. While many methods are now available for synthesizing amines containing aryl groups, the synthesis of amines containing alkyl groups still pose a challenge.
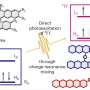
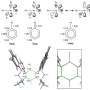
Now, the lab of Xile Hu at EPFL has developed a new method that can efficiently produce alkyl amines by using photocatalysis alongside copper catalysis. The photocatalysis allowed the chemists to start with alkyl redox-active esters instead of the usual alkyl halides, which are limited in terms of availability, stability, and are sometimes toxic.
The esters can be easily prepared from alkyl carboxylic acids, which are readily available, stable, and non-toxic. The copper catalysis was then used to link the alkyl unit generated in photocatalysis with a nitrogen-containing coupling partner to generate alkyl amines.
"Although metal-catalyzed cross coupling reaction has completely revolutionized the synthesis of aryl amines, it can hardly be used to synthesize alkyl amines," says Xile Hu. "The reason is that the necessary metal alkyl intermediates tend to decompose, and the alkyl-nitrogen ligation becomes difficult."
The work provides a new and efficient approach for the synthesis of alkyl amines, which has potential applications in the pharmaceutical and fine chemical industries. It offers high selectivity, compatibility with a large number of functional groups, and broad scope. In addition, the reactions are carried out at an ambient temperature, much milder than traditional methods. "And because many amino acids, natural products, and pharmaceuticals contain the alkyl carboxylic acid structural motif, our method can be used to rapidly functionalize these molecules," says Hu.
The researchers also provided over 50 examples where their new method is used for the synthesis of a diverse set of alkyl anilines with high chemoselectivity and functional group compatibility.
More information: Runze Mao et al. Decarboxylative C(sp3)–N cross-coupling via synergetic photoredox and copper catalysis, Nature Catalysis (2018). DOI: 10.1038/s41929-017-0023-z
Journal information: Nature Catalysis
Provided by Ecole Polytechnique Federale de Lausanne