This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking the potential of anti-perovskites through a convenient one-step synthesis route
Although perovskites have attracted a lot of attention lately, anti-perovskites hold just as much potential as functional materials. Bearing a similar crystal structure to perovskites but with an inverted electrical configuration, anti-perovskites exhibit peculiar properties that could be exploited, including negative thermal expansion, ionic conductivity, and even superconductivity. Unfortunately, thus far, synthesizing nanosized anti-perovskites has proven difficult, which is holding back these promising materials in catalytic applications.
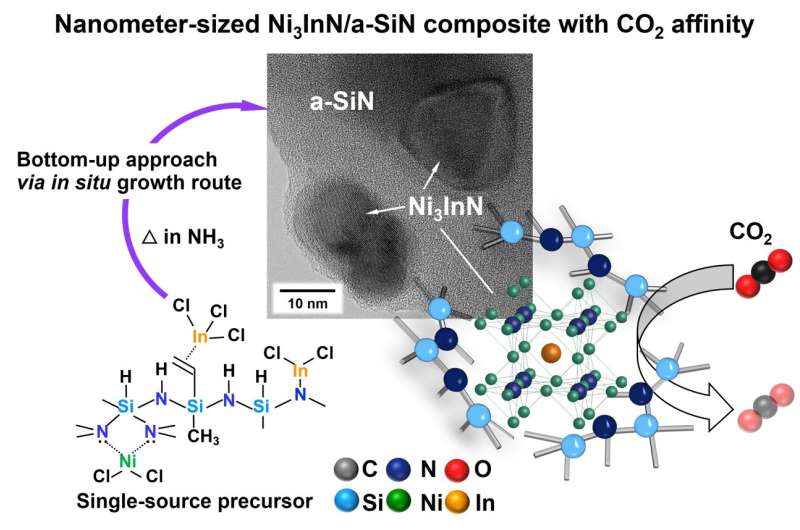
In a recent study published in the Journal of Materials Chemistry A, a research team led by Professor Yuji Iwamoto from the Department of Life Science and Applied Chemistry at Nagoya Institute of Technology, Japan, tackled the current challenges plaguing the synthesis of nitride-based anti-perovskites. They demonstrated a convenient synthesis technique to produce a nanocomposite material consisting of amorphous silicon nitride (a-SiN) ceramic with embedded nanometer-sized Ni3InN anti-perovskite crystals.
Their work was co-authored by Young International Faculty (Visiting Faculty) Shotaro Tada and Professor Ravi Kumar from the Department of Metallurgical and Materials Engineering at Indian Institute of Technology Madras (IIT Madras), India, and Director of Research Samuel Bernard from the University of Limoges, France.
The proposed synthesis method can be classified as a "Polymer-Derived Ceramics" (PDCs) route. First, polysilazane—used as a silicon nitride precursor—is chemically modified to include NiCl2 and InCl3 molecules. Then, through pyrolysis in an ammonia (NH3) atmosphere at a relatively low temperature of 300 °C, the modified precursor is transformed into the a-SiN matrix with embedded anti-perovskites in a single step.
"While our research team has previously developed a synthesis route for transition metal/a-SiN nanocomposites using polysilazanes modified with transition metal chlorides, this recent study presents a novel approach adopting multiple metal species, resulting in the in situ growth of Ni3InN intermetallic nanoparticles within an amorphous matrix," says Prof. Iwamoto.
Dr. Tada elaborates, "During the initial stages of the research, we encountered difficulties in obtaining a single-phase Ni3InN compound through stoichiometric blending. Through systematic analysis using different types of polysilazanes and spectroscopic studies, we found that the presence of vinyl groups in polysilazanes weakened the interaction with InCl3 due to steric hindrance, leading to enhanced migration of In sources into pre-formed Ni nanoparticles at lower temperatures. We addressed this problem by adding excess InCl3, resulting in the formation of a single phase Ni3InN."
This bottom-up synthesis strategy has several strengths. First, the resulting nanocomposite material is highly microporous and contains abundant interfaces between Ni3InN and the a-SiN matrix. Thus, it enables the modification of the electronic structure of the surfaces of the anti-perovskite nanoparticles formed in situ. Additionally, as a single-step low-temperature method, it provides a straightforward route to obtain complex, highly functional materials.
The researchers also showcased, as a proof of concept, the ability of the a-SiN/Ni3InN composite to adsorb and desorb CO2, which could be key to activating and transforming small molecules into value-added compounds for clean energy applications.
"This type of nanocomposite, with its diverse multi-metal composition, exhibits promising potential for heterogeneous catalyst design. By providing structural diversity and modifiability, it could facilitate the discovery of new catalytic functionalities," remarks Dr. Bernard.
The researchers hope that these exciting findings pave the way to versatile materials with sustainable applications.
More information: Shotaro Tada et al, An in situ growth route towards anti-perovskite Ni3InN nanoparticles embedded within amorphous silicon nitride, Journal of Materials Chemistry A (2023). DOI: 10.1039/D3TA06212K
Journal information: Journal of Materials Chemistry A
Provided by Nagoya Institute of Technology