This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel two-step electrolysis of water proposed for hydrogen production
A research team led by Prof. Chen Changlun from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, together with collaborators, developed advanced cobalt-doped nickel hydroxide bipolar electrodes and non-noble metal catalysts, which significantly improved the efficiency and stability of two-step water electrolysis for hydrogen production.
The results were published in the Chemical Engineering Journal and the Journal of Colloid and Interface Science.
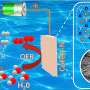
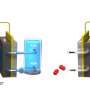
Traditional alkaline electrolyzers face problems such as mismatch with fluctuating renewable energy sources and hydrogen/oxygen mixing under high pressure, which limit their applications. Two-step water electrolysis overcomes these problems by completely separating hydrogen and oxygen production in time and space using a bipolar electrode, eliminating the need for a costly membrane separator.
The key is to develop high-performance bipolar electrode materials and efficient cell designs. However, commonly used nickel hydroxide electrodes have limitations in terms of electric buffering capacity and charging/discharging stability.
The researchers used a one-step electrodeposition method to fabricate cobalt-doped flexible nickel hydroxide bipolar electrodes on carbon cloth. Cobalt doping improved conductivity and electronic storage performance, and prevented parasitic oxygen production during hydrogen production.
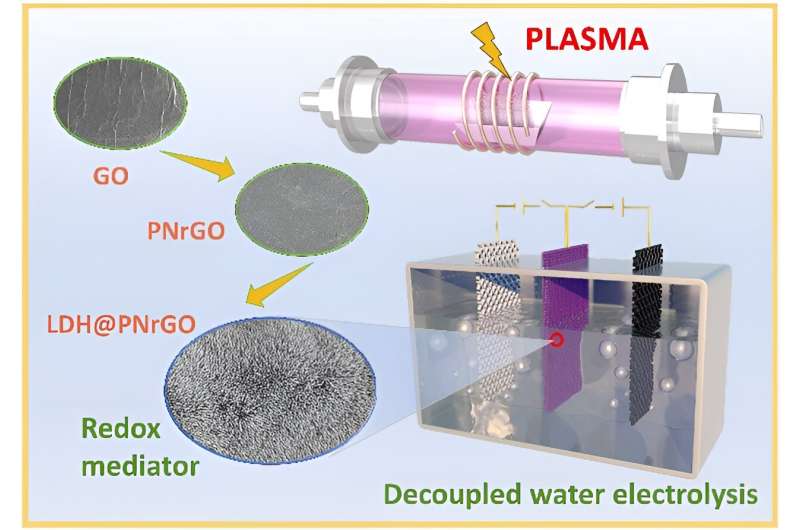
They also developed non-noble metal catalysts, including molybdenum-doped nickel-cobalt phosphide and plasma-induced iron composite cobalt oxide bifunctional electrodes, which showed high durability and activity. These electrodes enabled hydrogen and oxygen production at different times and locations by switching the current direction, resulting in low cell voltages, high decoupling efficiency, and high energy conversion efficiency.
To improve layered double hydroxide (LDH) electrodes, which suffer from limited capacity and poor conductivity/stability, the researchers used non-thermal plasma technology to fabricate nitrogen-doped nickel-cobalt LDH and nitrogen-doped reduced graphene oxide/nickel-cobalt LDH electrodes, which significantly improved capacity and conductivity.
Two-step water electrolysis shows promise for large-scale hydrogen storage and applications such as 5G base stations and data centers. "Our performance indicators for two-step water electrolysis for hydrogen production are synchronized with advanced indicators globally, marking an important step towards industrial operation," said Prof. Chen Changlun.
More information: Chengwei Sun et al, Precise surface reorganization on iron incorporated cobalt oxides/phosphides using plasma technology for alkaline water/seawater electrolytes, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.147374
Yuan He et al, High buffering capacity cobalt-doped nickel hydroxide electrode as redox mediator for flexible hydrogen evolution by two-step water electrolysis, Journal of Colloid and Interface Science (2023). DOI: 10.1016/j.jcis.2023.06.102
Journal information: Chemical Engineering Journal
Provided by Chinese Academy of Sciences