This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists sustainably create peptide-based medicines, pave way for greener cancer treatments
Scientists from the University of Manchester have uncovered a more efficient and sustainable way to make peptide-based medicines, showing promising effectiveness in combating cancers.
Peptides are comprised of small chains of amino acids, which are also the building blocks of proteins. Peptides play a crucial role in our bodies and are used in many medicines to fight diseases such as cancer, diabetes, and infections.
They are also used as vaccines, nanomaterials and in many other applications. However, making peptides in the lab is currently a complicated process involving chemical synthesis, which produces a lot of harmful waste that is damaging to the environment.
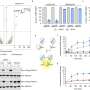
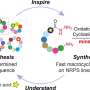
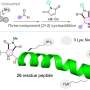
In a new breakthrough, published in the journal Nature Chemical Biology, Manchester scientists have discovered a new family of ligase enzymes—a type of molecular glue that can help assemble short peptide sequences more simply and robustly, yielding significantly higher quantities of peptides compared to conventional methods.
The technology could revolutionize the production of treatments for cancer and other serious illnesses, offering a more effective and environmentally friendly method of production.
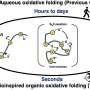
Professor Jason Micklefield, who led the team at The University of Manchester, said, "Using our new ligase enzymes we can produce peptides with promising anti-cancer activity in a single process with excellent yields. Previously, these types of peptides were produced in much lower yields, by a very laborious 10–12 step chemical synthesis process. By combining different ligases together in a single cascade reaction, we can make many different peptides."
For many years, scientists have been working on new ways to produce peptides. Most existing techniques rely on complex and heavily protected amino acid precursors, toxic chemical reagents, and harmful volatile organic solvents, generating large amounts of hazardous waste. The current methods also incur high costs, and are difficult to scale up, resulting in limited and expensive supplies of important peptide medicines.
The team in Manchester searched for new ligase enzymes involved in the biological processes that assemble natural peptides in simple bacteria. They successfully isolated and characterized these ligases and tested them in reactions with a wide range of amino acid precursors. By analyzing the sequences of the bacterial ligase enzymes, the team identified many other clusters of ligases likely involved in other peptide pathways.
The study provides a blueprint for how peptides, including important medicines, can be made in the future.
Dr. Guangcai Xu, who also worked on the project said, "The ligases we discovered provide a very clean and efficient way to produce peptides. By searching through available genome sequence data, we have found many types of related ligase enzymes. We are confident that using these ligases we will be able to assemble longer peptides for a range of other therapeutic applications."
Following the discovery, the team will now optimize the new ligase enzymes, to improve their output for larger scale peptide synthesis. They have also established collaborations with a number of the top pharmaceutical companies to help with rolling out the new ligase enzyme technologies for manufacturing future peptide therapeutics.
More information: Guangcai Xu et al, Cryptic enzymatic assembly of peptides armed with β-lactone warheads, Nature Chemical Biology (2024). DOI: 10.1038/s41589-024-01657-7
Journal information: Nature Chemical Biology
Provided by University of Manchester