This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Targeted protein degradation: New adapter molecule expands potential of cell's waste disposal system
Disease-causing proteins can be removed from the cell through targeted protein degradation. For this, the protein must be connected to one of the approximately 600 endogenous so-called ubiquitin ligases. So far, clinical success has only been achieved with two of these ligases.
A team of researchers at CeMM, led by Georg Winter, has now discovered an adapter for a new ligase, potentially significantly expanding the range of medical applications. Furthermore, the team managed to elucidate the molecular mechanism of this adapter, which could lead to a new general strategy for targeted protein degradation. The study was published in Nature Communications.
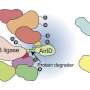
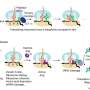
Using a drug to target any protein in the cell and remove it by the body's disposal system—this is, roughly speaking, how targeted protein degradation works. The drug is a small molecule that establishes a connection between the protein to be degraded and a so-called E3 ligase, which initiates the degradation process.
Two different strategies have been used so far: either the small molecule acts as a sort of glue, altering the surface of the ligase or the protein so they bind together. Or it functions as an adapter with two binding sites, one for the ligase and one for the protein. The glues are referred to as "Molecular Glue Degraders" (MGDs) and the adapters as "Proteolysis Targeting Chimeras" (PROTACs).
Approximately 600 different E3 ligases are known in the human body, but all drugs for targeted protein degradation currently under clinical investigation use the same two E3 ligases. This has the consequence, particularly in the use of these agents against cancer, that resistance can develop.
Additionally, the two ligases used are produced almost everywhere in the body, complicating tissue-specific or cell-type-specific application of the agents. Lastly, not all proteins can be degraded by these two ligases, likely due to their surface structure.
A small molecule with many advantages
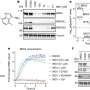
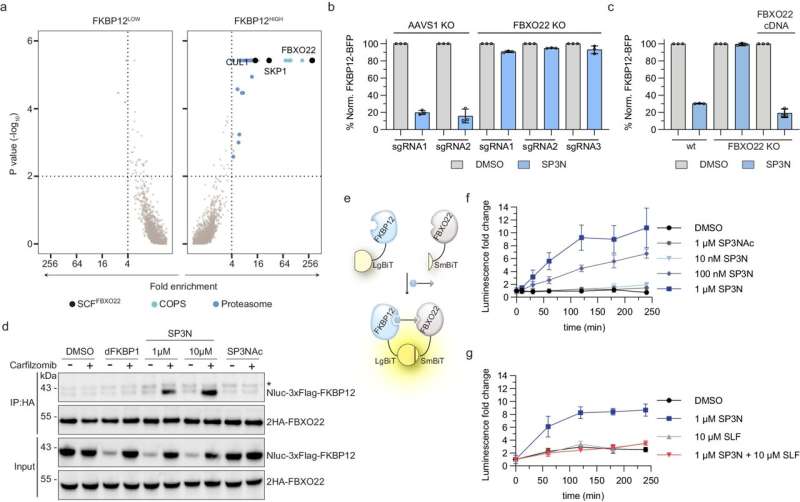
Recruiting new ligases for targeted protein degradation is therefore an urgent necessity for developing new therapies, especially against cancer, and for biomedical research. Winter's team has now succeeded in this endeavor: In their study, they presented an adapter molecule that recruits a new E3 ligase named FBXO22 for protein degradation.
Additionally, the researchers elucidated the mechanism of action of this molecule, which, while acting similarly to PROTACs, has specific properties that could lead to a new strategy in the systematic development of agents for targeted protein degradation.
The molecule, named SP3N, consists of a protein-binding part, the so-called ligand, and a carbon chain attached to it, ending with a nitrogen compound (amine). This chain, known as an alkylamine, can recruit a new E3 ligase because it is converted in the body into an aldehyde, which can chemically bind to the E3 ligase FBXO22.
"Compared to classical PROTACs, this new class of agents is significantly smaller, which will bring advantages in further development," says study leader Winter.
"Furthermore, the E3 ligase FBXO22 and the enzymes that metabolize it are produced in large quantities in various tumor types. This opens up new ways to specifically target protein degradation to tumor cells," adds first author Chrysanthi Kagiou.
Through these advantages, the researchers hope to increase the effectiveness of this therapeutic strategy while simultaneously avoiding potential side effects in healthy tissue.
More information: Chrysanthi Kagiou et al, Alkylamine-tethered molecules recruit FBXO22 for targeted protein degradation, Nature Communications (2024). DOI: 10.1038/s41467-024-49739-3
Journal information: Nature Communications
Provided by CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences