This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists show that Urm1 protects proteins in stress situations
To prevent proteins from being damaged during cellular stress, they are concentrated in so-called stress granules. Scientists from the department of Cellular Biochemistry at the Max Planck Institute of Biochemistry have now been able to show for the first time that the protein Urm1 has a critical role in this process.
In yeast cells, the ubiquitin-like protein facilitates the onset of phase separation and thus the formation of stress granules. The results of the study were published in the journal Cell.
Cells may experience various conditions of stress, such as heat stress during a fever. Such conditions can damage proteins—the molecules responsible for almost every process of life. The ability of cells to mount a defensive stress response is crucial for their survival.
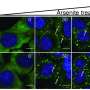
As part of this response, the small protein ubiquitin is attached to damaged proteins to signal that they should be removed by degradation. Other proteins, however, must be protected during stress by concentrating them in so-called stress granules, via a process called phase separation.
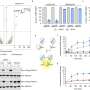
How this works exactly is not yet well understood. In a recent study, Lucas Cairo, Sae-Hun Park and their colleagues describe the role of the small protein Urm1 in regulating formation of reversible condensates under stress.
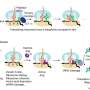
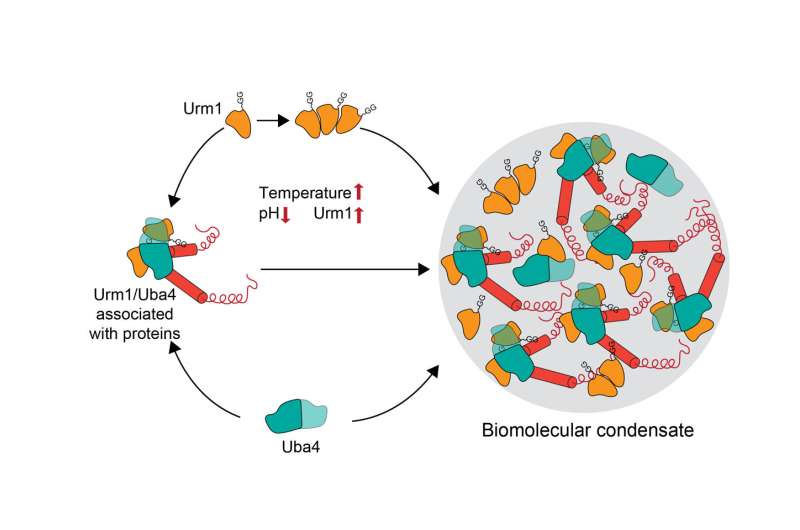
Urm1 is related to ubiquitin and like ubiquitin can be covalently attached to target proteins. However, unlike ubiquitin, the cellular role of Urm1 had not been fully understood. The researchers found that stress triggers the covalent attachment of Urm1 to target substrates, promoting their assembly into stress granules and other biomolecular condensates. This enables their safe storage until the stress subsides.
More specifically, Urm1 facilitates the initiation of phase separation by enhancing protein-protein interactions conducive to this biophysical process. Key to this is the intrinsic ability of Urm1 to sense acidification of the cellular milieu that occurs upon stress. As a result, Urm1 self-interacts and associates with other proteins through multivalent interactions, forming a complex protein interaction network.
This network facilitates the deposition of proteins within condensates located in both the cytoplasm and cell nucleus. Attachment of Urm1 to target proteins is facilitated by the enzyme Uba4, which coassembles with Urm1 in the condensates. In the absence of Urm1, cells can no longer cope with stress. These findings identify Urm1 as a ubiquitin-like protein with a critical function in the cellular stress response.
More information: Lucas V. Cairo et al, Stress-dependent condensate formation regulated by the ubiquitin-related modifier Urm1, Cell (2024). DOI: 10.1016/j.cell.2024.06.009
Journal information: Cell
Provided by Max Planck Society