This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New fluorescent reporting system illuminates accumulation of unfolded proteins in endoplasmic reticulum
The endoplasmic reticulum (ER) is a major organelle responsible for protein folding and trafficking. Protein folding stress in the ER can lead to the accumulation of unfolded or misfolded proteins, known as ER stress, which activates the unfolded protein response (UPR). However, UPR can also be activated in the absence of ER stress. Deciphering the complex relationship between ER stress and UPR is critical to understanding cellular stress responses in physiological and pathological conditions.
In a study published in Cell Reports on June 11, researchers led by Prof. Wang Likun from the Institute of Biophysics of the Chinese Academy of Sciences have developed a novel fluorescence reporting system that allows researchers to visualize the accumulation of unfolded proteins within the ER lumen in living cells in real time.
Through this system, researchers can better understand the intricate relationship between ER stress and UPR, and reveal the dynamic changes in ER protein homeostasis under various physiological and pathological conditions.
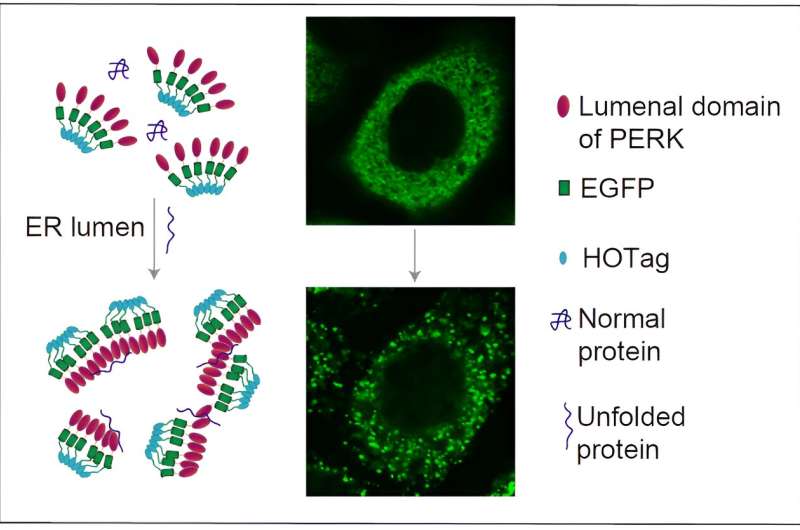
In this study, the researchers fused the ER luminal domain of the ER stress signaling protein PERK (PERK(LD) with green fluorescent protein (EGFP) and a peptide segment that tends to form hexamers (HOTag) to create a fluorescent reporting system.
Upon binding to unfolded proteins, this system rapidly and reversibly forms fluorescent spots that can be visualized by confocal microscopy. This system showed high sensitivity and reversibility in HeLa and COS-7 cells, providing real-time insights into the dynamic changes of ER stress and UPR.
Using various techniques, including co-localization experiments and immunoprecipitation, the researchers validated the direct binding between the fluorescent reporting system and unfolded proteins. They demonstrated that the system could quantitatively detect the accumulation of unfolded proteins in the ER and displayed a dose-response relationship at different concentrations.
To further elucidate the relationship between ER stress and UPR, the researchers examined the differential UPR responses to the anti-cancer drug Bortezomib in different cell types, revealing the complexity of how Bortezomib regulates UPR and unfolded protein accumulation by different mechanisms in different cell types.
This study unravels the complex interactions between ER stress and UPR, providing a powerful tool for understanding how these processes operate in health and disease. This discovery provides a new perspective for the development of novel therapeutic strategies, particularly in diseases involving ER stress, such as neurodegenerative diseases, diabetes, and cancer.
More information: Fenfen Xu et al, Deciphering ER stress-unfolded protein response relationship by visualizing unfolded proteins in the ER, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.114358
Journal information: Cell Reports
Provided by Chinese Academy of Sciences