This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The worm has turned: DIY lab platform evaluates new molecules in minutes
Plants are powerhouses of molecular manufacturing. Over the eons, they have evolved to produce a plethora of small molecules—some are beneficial and valuable to humans, while others can be deadly. For years, a good way for scientists looking for new medicines to distinguish beneficial plant-derived molecules from harmful ones has been through a scientific sniff test—dab a bit of the molecule at one end of a petri dish and drop tiny nematode worms (C. elegans) at the other, then wait to see if the chemically sensitive worms move toward or away from the compound in question, a process known as chemotaxis.
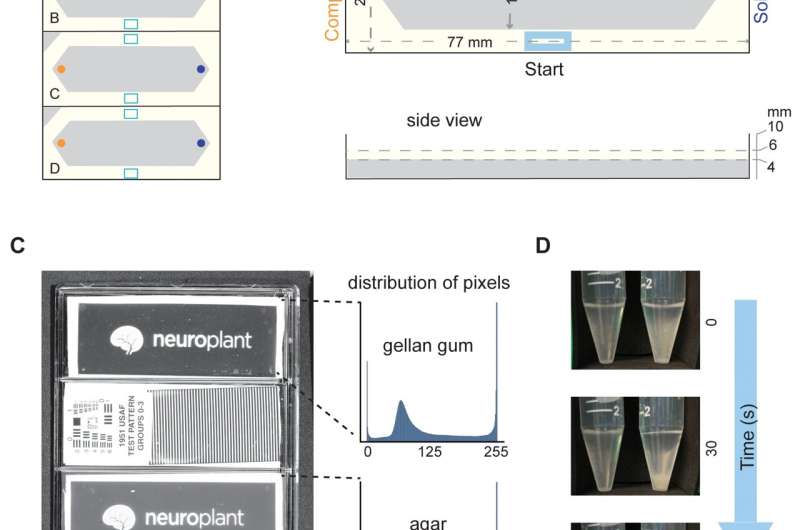
This "artisanal" method is achingly slow. It can take two hours to assay a single new molecule. But now a team at the Wu Tsai Neurosciences Institute at Stanford University, led by Miriam Goodman, a professor of molecular and cell biology, has developed hardware and software that turns an off-the-shelf flatbed scanner into a lab platform that can evaluate dozens of chemotaxis samples in minutes. The platform can prep 20 plates at a time and fit four plates on a scanner to perform 80 chemical assays in about an hour.
"In the artisanal days, if someone were really skilled and had done all the prep work and had all the materials, it might take two weeks to do that many assays," Goodman said. The team describes the components of their DIY platform and offers the open-source code in a new paper in the journal PLOS Biology.
The big idea
It sounds simple, but developing the platform has been anything but for the team, which included co-investigators Seung "Sue" Y. Rhee, Director of the Plant Resilience Institute at Michigan State University (formerly of the Carnegie Institution for Science at Stanford); and Thomas R. Clandinin, professor of neurobiology at Stanford. It took the trio and their labs more than five years to design, create, test, and evaluate their approach from inception to publication.
The project, dubbed the "Neuro-Plant Initiative," required experts in neurosciences, animal and plant biology, laboratory sciences, mechanical engineering, and computer science. The team now hopes the platform will become ubiquitous and lead to rapid discovery of promising new molecules for use in medical, biology, agricultural, and neurosciences labs currently mired in artisanal methods.
"In the nematode research community, there are roughly 1,200 labs across the world," Goodman noted. The platform could prove useful for discovering other kinds of chemicals or identifying which species of bacteria attract or repel nematodes that eat bacteria, she explained.
Myriad possibilities
Co-author Sue Rhee, who is MSU Foundation Professor in the Departments of Biochemistry & Molecular Biology, Plant Biology, and Plant, Soil & Microbiology studies how plants make myriad compounds and use them to communicate with their environments. Of the hundreds of thousands of compounds that plants produce, Rhee says we know very little about how they are made or used in nature.
"A long-standing dogma is that they are used to defend against pests or to attract pollinators, but only a tiny number of these compounds have known roles," Rhee said. "Through this ingenious high-throughput chemotaxis assay, I hope that we can start to unravel these chemical mysteries at scale."
Co-author Thomas Clandinin is a neurobiologist who studies how animals use specific sensory inputs to select appropriate behavioral actions. His lab contributed to the development of a way to stabilize worms when suspended in liquid, facilitating automated methods for dispensing worms at the scale needed to move away from the artisanal method.
"Developing this automated method to examine chemotaxis behavior at scale will open a host of new possibilities for exploring the rich connections between odors and their receptors," Clandinin said.
Next steps
Next, Goodman hopes to put their invention to work in her own neurosciences lab to understand the neural and chemical basis of the nematodes' ability to distinguish good molecules from bad. She is pursuing a collaboration with researchers at Harvard University to use calcium imaging to record the activity of all the worm's olfactory—or smell-sensitive—neurons as it is moving toward or away from a molecule. In that work, Goodman plans on making use of her assay platform to do behavioral research at a greater scale, manipulating individual neurons in the worms' nervous systems and eventually matching compounds to receptors.
The nematodes' sniff test is a skill shared by many animals, including humans, Goodman points out. She hopes to apply these learnings in neuroscience to that intriguing subject, work she is certain will be accelerated by this new platform. Whether in worms or humans, she says, the chemical receptors in the olfactory system binding to these molecules are holding on to the exact same chemical.
"The shape of those binding pockets ought to be similar in both beings. Even if they are dissimilar, it will be helpful to know that," Goodman said. "That is research I'm really excited about."
More information: Emily Fryer et al, A high-throughput behavioral screening platform for measuring chemotaxis by C. elegans, PLOS Biology (2024). DOI: 10.1371/journal.pbio.3002672
Journal information: PLoS Biology
Provided by Stanford University