This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reveal crucial role of RNA in modulating autophagic degradation of protein condensates
A research group led by Prof. Zhang Hong from the Institute of Biophysics of the Chinese Academy of Sciences has demonstrated that RNAs act as a switch to change the fate of phase-separated protein condensates from autophagic degradation to stress-triggered accumulation. The study was published online in Journal of Cell Biology.
Autophagy is a lysosome-mediated degradation process. Protein condensates assembled via liquid–liquid phase separation (LLPS) can be effectively degraded by autophagy. However, they can evade autophagic degradation under stress or pathological conditions to confer stress resistance or contribute to the pathogenesis of various diseases.
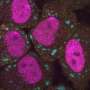
For example, previously studies by Prof. Zhang's lab found that the oocyte-derived PGL proteins (PGL-1 and PGL-3) are removed by autophagy in somatic cells during C. elegans embryogenesis under normal growth conditions (15–25°C). Degradation of PGL-1/-3 requires the receptor protein SEPA-1 and the scaffold protein EPG-2. In embryos laid under heat stress conditions, however, PGL proteins escape autophagic degradation, and accumulate into a large number of granules to confer stress resistance.
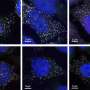
In this study, the researchers found that PGL granules formed under heat stress conditions contain proteins involved in mRNA metabolism such as the eIF4E homolog IFE-1. RNA-FISH assays revealed that RNAs are also sorted into PGL granules in heat-stressed embryos.
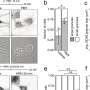
Then, the researchers found that sorting of mRNA into PGL granules modulates their autophagy degradation, and that depleting factors involved in mRNA processing, transport and translation reduces the recruitment of mRNA into PGL granules and promotes their autophagic degradation, while impaired mRNA degradation increases the recruitment of mRNA to PGL granules and promotes their accumulation.
PGL-1/-3/SEPA-1 proteins undergo LLPS to assembly into PGL condensates. EPG-2 promotes the liquid-to-gel like transition of PGL condensates, which is essential for their autophagic degradation. The researchers demonstrated that mRNA promotes LLPS and maintains the liquidity of PGL condensates, and also inhibits the recruitment of EPG-2. They also found that factors affecting the recruitment of mRNAs into PGL granules modulate the co-localization of EPG-2 and PGL granules in heat-stressed embryos. These results suggested that mRNAs modulate autophagic degradation of PGL granules by inhibiting the recruitment of the gelation-promoting scaffold protein EPG-2.
This study reveals a crucial role of RNA in switching the fate of phase-separated protein condensates from autophagic degradation to stress-triggered accumulation, providing novel insights into the accumulation of ribonucleoprotein aggregates associated with the pathogenesis of various diseases.
More information: Hui Zheng et al, RNA recruitment switches the fate of protein condensates from autophagic degradation to accumulation, Journal of Cell Biology (2023). DOI: 10.1083/jcb.202210104
Journal information: Journal of Cell Biology
Provided by Chinese Academy of Sciences