This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists show ribosomes play an unexpected role in blood vessel formation
Angiogenin, an enzyme that plays a critical role in cellular stress responses and aids vascular formation, has previously been implicated in the formation of solid cancer tumors, neurodegenerative disorders and epigenetic inheritance, and has been the focus of intense study by scientists for four decades.
Research by Andrei Korostelev, Ph.D., and Anna Loveland, Ph.D., at UMass Chan Medical School, shows that the ribosome plays an unexpected role in its function by activating angiogenin to cleave transfer RNA (tRNA), thereby halting protein production.
Published in the journal Nature, these findings uncover a unique role of the ribosome in angiogenic functions, which may have important implications for the design of cancer therapeutics and neurodegenerative disease treatments.
"Elucidating the function and therapeutic potential of angiogenin requires a precise understanding of the molecular mechanism involved in its activation," said Dr. Korostelev, professor of RNA therapeutics and study author. "Our work answers a long-standing question regarding angiogenin activation. We show that the ribosome binds to and changes the shape of the enzyme so it can cleave tRNA.
"These findings open the door to translational investigation that will hopefully explore the therapeutic potential of targeting the ribosome-binding of angiogenin to treat cancer and neurodegenerative disorders," added Korostelev.
A ribonuclease that cleaves RNA, angiogenin was characterized in 1985 as the first enzymatic factor known to stimulate angiogenesis—the formation of blood vessels. Revolutionary at the time because it supported the theory that cancer cells facilitate vascular growth to support themselves, this discovery spawned decades of subsequent studies on angiogenesis in cancer treatment, wound healing, regeneration, organ development and neurodevelopment.
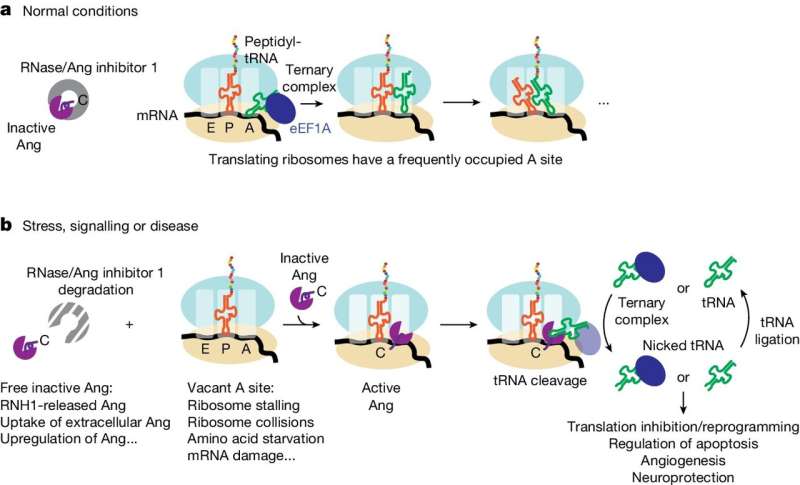
Though it was understood that angiogenin could inhibit protein synthesis by cleaving tRNA in cells, laboratory experiments paradoxically found only very low levels of cleavage activity with purified angiogenin. This puzzled researchers, who were unable to identify the mechanisms through which angiogenin was being activated, leading some to believe that other, unidentified cellular components played a strong role in angiogenin activation.
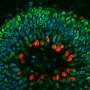
"Angiogenin is the protein that determines vascular formation in vertebrates," explained Dr. Loveland, assistant professor of RNA therapeutics and co-author of the study. "When cells are stressed and hypoxic, angiogenin is activated to form new blood vessels to provide oxygen.
"Likewise, cancer cells use mechanisms that stimulate angiogenin to form new blood vessels that can then feed tumors. In the brain, angiogenin has been shown to be neuroprotective. Mutations in angiogenin, for instance, are associated with amyotrophic lateral sclerosis.
"Despite its fundamental biological importance and intense study, scientists haven't been able to pinpoint how angiogenin was turned on—until now."
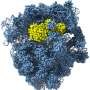
Using the latest cryo-EM technology, Korostelev and Loveland were investigating the structure of the ribosome when they began observing an unidentified protein in a ribosome sample that they obtained from their collaborators in the laboratory of Allan Jacobson, Ph.D., chair emeritus and professor of microbiology & physiological systems at UMass Chan.
Cryo-EM is a type of electron microscopy that uses cryogenic temperatures to freeze cell samples and hit them with an electron beam. As electrons pass through the cell samples, images are captured that can be reconstructed into a high-resolution 3D model.
An indispensable tool for studying the structures of biological molecules, cryo-EM allows scientists to observe the structure of biological molecules at an exceptional level of detail.
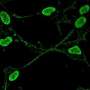
Ribosomes are large, complex machines found inside cells. These macromolecular machines are normally responsible for the synthesis of proteins. Ribosomes read the messenger RNA that corresponds to the genetic sequence of a gene and assemble the amino acids into the order needed to create the specified protein.
Under stress, however, ribosomes perform an additional, unsuspected role, according to Korostelev and Loveland.
"When we looked at the images of the ribosome, we could tell something was off," said Loveland. "There was a protein binding to our ribosomes for which we couldn't account—likely a ribonuclease, from its shape and how it was folded. It resembled angiogenin."
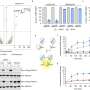
Using chemical assays, Loveland was able to confirm their suspicions. In times of cellular stress, ribosomes bind and activate angiogenin.
In nonstressful situations, angiogenin is typically bound to an inhibitor protein. Meanwhile, the ribosome is busy assembling the amino acids needed to build proteins. tRNA brings an individual amino acid to the ribosome's amino-acyl site (A site) where it joins with other amino acids to produce a protein.
During times of stress, however, something different happens. Angiogenin disassociates from its inhibitor and translation slows, leaving the ribosome's A site unoccupied. Angiogenin steps into this void, binding to the A site and turning on its nuclease activity allowing it to nick the next incoming tRNA. These nicks inhibit tRNA function and subsequent protein synthesis, eventually regulating multiple cellular functions, such as blood vessel formation.
"The translation of genes into proteins is changed by this cleaving," said Korostelev. "These findings add to our understanding of how angiogenin is activated and provides an important new therapeutic target and downstream effects to investigate."
More information: Anna B. Loveland et al, Structural mechanism of angiogenin activation by the ribosome, Nature (2024). DOI: 10.1038/s41586-024-07508-8
Journal information: Nature
Provided by University of Massachusetts Medical School