This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists develop chameleon-like molecular chain with dual ion binding capability
Everyday objects such as metal chains, handcuffs, and key rings are examples that demonstrate a unique combination of properties where hard, rigid rings are interlocked together to exhibit flexibility and strength as a whole, and as such enable them to perfect their intended functions.
Molecules composed of interlocked, nano-sized rings are known as catenanes, which are promising candidates for the development of molecular switches and machines. Yet, due to their challenging synthesis, applications of catenanes in other areas are largely unexplored.
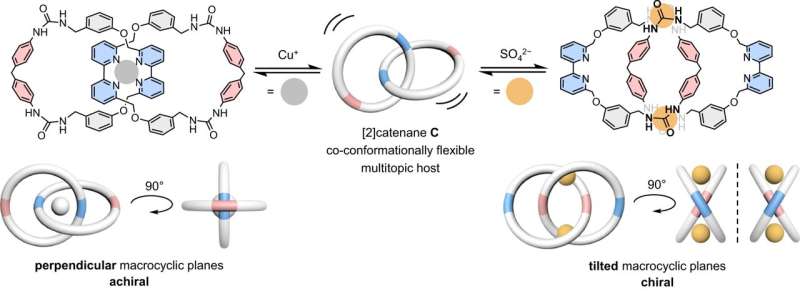
Recently, a research team led by Professor Ho Yu Au-Yeung from the Department of Chemistry at The University of Hong Kong (HKU) has synthesized a catenane composed of two freely-rotating rigid macrocycles and showed that the catenane can bind strongly and selectively to either a copper(I) cation or a sulfate anion despite their opposite charge and different geometry.
The ability to detect and differentiate these specific ions has important implications for applications in areas such as environmental monitoring and medical diagnostics. The work has recently been published in Nature Communications.
As same charges repel and opposite charges attract each other, a binding site that attracts a positively charged cation will normally experience a repulsive interaction with the negatively charged anion and vice versa, which made designing a host that is suitable for both cation and anion very challenging.
To overcome this, the team installed both cation and anion binding sites on each of the interlocked rings, and by virtue of the rotatory motions of the catenane, the host can efficiently adjust the relative position of the binding sites and freely adapt a specific form favorable for the spherical copper(I) cation or the tetrahedral sulfate anion, resembling a chameleon that can change its appearance to fit in specific environments.
Apart from their industrial and environmental significance, both copper(I) and sulfate ion are essential for proper cell growth and organism development. The strong and selective binding to these ions by the catenane host could hence be exploited for the extraction and recycling of these ions from environmental samples.
Also, just as the measurement of sodium ions, chloride and other electrolytes in blood samples can be a routine test for blood pressure monitoring and general health, new technologies for selective recognition and binding of ions and minerals will be useful for diagnostic and therapeutic purposes.
"This work highlights catenane as an efficient candidate for potent molecular receptors with versatile structures, switchable properties and guest binding behaviors," said Professor Au-Yeung.
In terms of future plans, Professor Au-Yeung and his group are developing more sophisticated catenane hosts for the simultaneous binding of multiple cations, anions and ion pairs.
More information: Yueliang Yao et al, Dynamic mechanostereochemical switching of a co-conformationally flexible [2]catenane controlled by specific ionic guests, Nature Communications (2024). DOI: 10.1038/s41467-024-46099-w
Journal information: Nature Communications
Provided by The University of Hong Kong