The asymmetric synthesis of halogenated compounds from carboxylic acids is world first
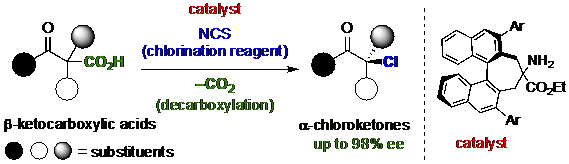
Toyohashi University of Technology researchers led by Associate Professor Shibatomi developed new catalytic reaction to produce chlorine-containing organic molecules in isomerically pure (left- or right-handed) form
Molecules don't have hands, but some of them are left- or right-handed. Many chemical compounds display a feature called chirality, where two versions—known as enantiomers—exist for the same molecule. Although their atoms are connected in exactly the same sequence, the two enantiomers are distinct mirror images, like a pair of hands.
Enantiomers can have very different properties. For example, only the right-handed form of glucose gives you energy—the left-handed isomer cannot be metabolized, even though it tastes the same. Many pharmaceuticals are also chiral, and often only one enantiomer has a medicinal use. Therefore, chemists working on complex molecules have developed a variety of tricks to guarantee isomer purity. However, for some reactions this remains a challenge.
Now, the research team has developed a reaction to produce an important class of compounds in pure left- or right-handed form. Organohalides are molecules in which a halogen, such as chlorine, is bonded to carbon. Many are found in nature, or used in medicine. They can be produced from another family of compounds, carboxylic acids, by simply replacing an acid with a halogen. Unfortunately, if the target compound is chiral, this substitution produces left- and right-handed isomers in equal amounts.
The Toyohashi University of Technology research team solved this problem by catalyzing the reaction with a catalyst that is itself chiral. Nowadays, catalysts come in a wide range of shapes and sizes—often rivalling the complexity of the actual target molecule. "We screened a diverse array of chiral catalysts, such as Lewis acid, Brønsted acid, and Lewis base catalysts," study lead author Kazutaka Shibatomi says. "Finally, we found an amine that gave us organohalides with up to 98% enantiomeric purity - even though our starting material was a 50/50 mixture."
The chlorinated products, known as chloroketones, are building blocks for more important chiral molecules like pharmaceuticals. Because chlorine is only weakly bonded to carbon, it can be easily substituted by another atom to make a new molecule. Using one of the many compounds produced in enantiomeric purity by their new reaction, the research team synthesized Cathinone, a natural stimulant.
"The substitution proceeds in a simple, classic way," Associate Prof. Shibatomi says. "While chlorine leaves the molecule on one side, the incoming group approaches from the opposite side. The product's chirality just depends on the arrangement of these atoms, so if you begin with a pure enantiomer, you retain that purity. This could open up a whole class of compounds that were previously a major challenge to produce as pure enantiomers."
More information: Kazutaka Shibatomi et al, Enantioselective decarboxylative chlorination of β-ketocarboxylic acids, Nature Communications (2017). DOI: 10.1038/ncomms15600
Journal information: Nature Communications
Provided by Toyohashi University of Technology