Credit: Wiley
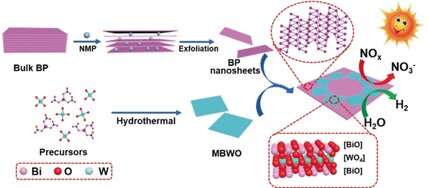
A significant advance in the photocatalytic activity of conventional materials is demonstrated by a two-dimensional heterostructure comprising nanolayers of two semiconductors: black phosphorus and bismuth tungstate. As researchers have reported in the journal Angewandte Chemie, this catalyst harnesses the energy of visible light to split water and produce hydrogen, and to break down nitrogen monoxide in exhaust gas.
Just as plants use photosynthesis, certain semiconductors are able to absorb the energy of light and use this to power chemical reactions. For example, bismuth tungstate (Bi2WO6) should, in principle, be suitable for the photocatalytic degradation of nitrogen monoxide (NO) and the production of hydrogen. However, results so far have not been very satisfactory. One approach to improving the performance of this material is to bind two-dimensional nanolayers of the bismuth tungstate into a layered heterojunction with a second nanolayer of a different semiconductor.
A team led by Dongyun Chen and Jianmei Lu at Soochow University, Suzhou, and Jiangsu University, Zhenjiang (China) found that black phosphorus may be a suitable partner for this type of heterostructure. This material demonstrates photocatalytic properties, though it has had limited application to date.
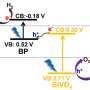
Black phosphorus consists of rippled layers of six-membered rings that can be split into individual atomic layers. The researchers covered these nanolayers evenly with 50 nm chips of bismuth tungstate. The two semiconductors are in very close contact in this simply and efficiently producible heterostructure, resulting in a synergetic effect. The black phosphorus provides a broad absorption range into the spectrum of sunlight. The energy levels of the electrons in the two materials are favorably placed. This allows the light-induced positive and negative charges (electron-hole pairs) to be efficiently separated, transported within the heterostructure, and transferred to molecules. The researchers propose that the charge-transfer mechanism resembles the so-called Z-scheme present in photosynthesis.
As expected, the photocatalytic degradation of NO by the heterostructure was significantly more effective than with other bismuth-based materials. For the photocatalytic production of hydrogen, an additional platinum-based co-catalyst was added. Under irradiation, electrons can move from the heterostructure to platinum atoms, and from there they are able to rapidly reduce the H+ ions in water to form hydrogen gas. With visible light, the efficiency of the catalytic process was nine times that of pure bismuth tungstate.
The researchers suggest that black phosphorus may have broad applicability that extends to renewable energies and treatment of exhaust gases.
More information: Jundie Hu et al, Z-Scheme 2D/2D Heterojunction of Black Phosphorus/Monolayer Bi2 WO6 Nanosheets with Enhanced Photocatalytic Activities, Angewandte Chemie International Edition (2019). DOI: 10.1002/anie.201813417
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley