This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists produce new-to-nature enzyme containing boron
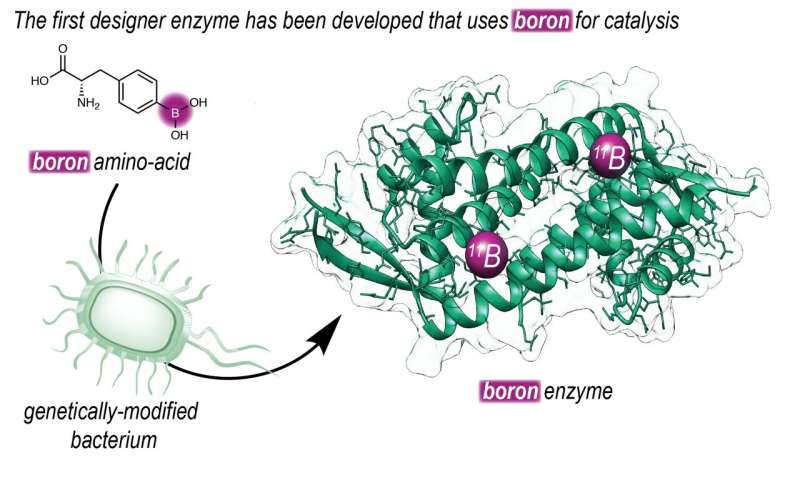
Boronic acid has been used in organic chemistry for decades, even though it is not present in any organism. "It gives rise to different chemical reactions than those we find in nature," explains Gerard Roelfes, Professor of Biomolecular Chemistry & Catalysis at the University of Groningen.
His group created an enzyme with boronic acid at its reactive center and then used directed evolution to make it more selective and to improve its catalytic power. Furthermore, enzymatic reactions are more sustainable than classical chemical reactions, as they take place at low temperatures and without toxic solvents. The study was published online in the journal Nature on 8 May.
The application of boron in organic chemistry dates back some seventy years and was awarded a Nobel Prize for Chemistry in 1979. In recent years, the interest in boron as a catalyst has grown, but as yet, its use in the chemical industry is limited.
Roelfes explains, "So far, boron catalysis is too slow and it is not very suitable for enantioselective reactions. These types of reactions are used to create chiral molecules, which can exist in two versions that are mirror images of each other, like a left and a right hand.
"In many drugs, both hands can have a different effect. It is, therefore, important to selectively produce the proper hand, especially for the pharmaceutical industry."
Expanded genetic code
"To make this possible, we set out to introduce boron into an enzyme. Our group has a long history of designing enzymes that don't exist in nature," says Roelfes. The Roelfes group used an expanded genetic code to introduce a non-natural amino acid that contains a reactive boronic acid group into an enzyme. "Using this technique, we can determine at the DNA level where we place the amino acid in a protein."
Once they made an enzyme with boronic acid at its reactive center, they could use directed evolution to increase its efficiency, resulting in faster catalysis. Roelfes adds, "Furthermore, by placing the boronic acid in the chiral context of an enzyme, we were able to achieve highly enantioselective catalysis. The reaction that is described is a proof of principle and shows the way to harnessing the catalytic power of boron in enzymes."
Biocatalysis
Using enzymes to create organic compounds is important for the pharmaceutical industry. "In their push towards greener and more sustainable ways of producing drugs, they are looking at biocatalysis to replace conventional chemical reactions," Roelfes says.
At the University of Groningen, concerted efforts are being made towards this goal. Roelfes concludes, "We have a number of research groups at the Faculty of Science and Engineering engaged in this kind of work, using different approaches to create biocatalytic solutions for the chemical industry."
In this context, Roelfes and his team will continue to develop their boronic acid enzymes and create other such new-to-nature enzymes.
More information: Gerard Roelfes, Boron catalysis in a designer enzyme, Nature (2024). DOI: 10.1038/s41586-024-07391-3. www.nature.com/articles/s41586-024-07391-3
Journal information: Nature
Provided by University of Groningen