This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method uses enantioselective nickel catalysis to synthesize multifunctional chiral alkylboron compounds
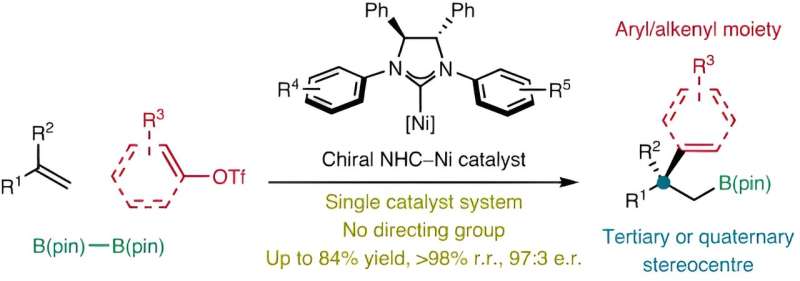
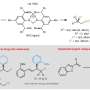
National University of Singapore (NUS) chemists have conceived a strategy using chiral nickel catalysts to facilitate the carboboration of alkenes, generating high-value enantioenriched alkylboronic esters.
Enantioenriched boronic esters are molecules that are widely used as building blocks in chemical synthesis to produce various organic compounds. Multicomponent transformations that produce these organoboron compounds using non-precious catalytic systems are highly sought-after but remain scarce. This is primarily due to the lack of a suitable chiral catalyst that can create these boronic esters with a high degree of efficiency and selectivity.
A research team led by Associate Professor Koh Ming Joo, from the Department of Chemistry, NUS has developed a new method that takes advantage of readily available nickel catalysts containing chiral and sterically bulky ligands known as N-heterocyclic carbenes (NHCs).
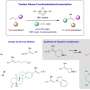
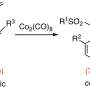
These catalysts play a crucial role in the reaction process by bringing together and facilitating the union of three different components: alkenes (molecules with carbon-carbon double bonds), organotriflates and a commercially available compound called bis(pinacolato)diboron.
This method is unique as there is no requirement for an additional directing group to guide the reaction. The reaction enables access to functionalized alkylboronates bearing tertiary or quaternary β-stereocenters with simultaneous control of regioselectivity and enantioselectivity.
The research was a collaboration with Professor Ma Jun-An and Associate Professor Nie Jing from Tianjin University, as well as Professor Shi Shi-Liang from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. Their findings were published in Nature Synthesis.
Prof Koh said, "Our studies show that these enantioselective nickel-catalyzed transformations operate through a series of steps involving carbonickelation followed by borylation, which are distinct from previously reported carboboration reactions."
"As detailed in the research paper, this methodology offers straightforward access to prized precursors that simplify the synthesis of complex bioactive molecules. We believe that our work will facilitate numerous applications in the field of stereoselective organic synthesis," added Prof Koh.
Looking ahead, the research team is actively designing chiral NHC-nickel catalysts to promote new classes of multicomponent alkene functionalization reactions that generate synthetically useful compounds.
More information: Xiaohua Luo et al, Enantioselective synthesis of multifunctional alkylboronates via N-heterocyclic carbene–nickel-catalysed carboboration of alkenes, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00492-x
Journal information: Nature Synthesis
Provided by National University of Singapore