This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Breakthrough in β-lactam synthesis using nickel catalysts
Led by Director Chang Sukbok, scientists from the Center for Catalytic Hydrocarbon Functionalizations within the Institute for Basic Science (IBS) have made a significant advancement in the synthesis of β-lactam scaffolds, which are structural components frequently found in essential antibiotics such as penicillins and carbapenems. This breakthrough overcomes challenges in β-lactam synthesis to promise streamlined pathways for drug development.
The core chemical structure that makes up penicillins is a four-membered cyclic amide scaffold called chiral β-lactam, which is also frequently found in other types of major antibiotics like carbapenems and cephalosporins. The high-value potential of chiral β-lactam has been recognized in modern science given such relevance to the pharmaceutical products, leading to many efforts to synthesize them using readily available raw chemicals.
Back in 2019, the IBS group unveiled a catalytic reaction that allowed access to chiral γ-lactams, five-membered amide structures that differ in the ring size from β-lactams. They managed to achieve high enantioselectivity by utilizing chiral iridium catalysts; however, the same approach could not be applied to the synthesis of the four-membered variant, β-lactam.
One major obstacle in accessing β-lactams was, in fact, the outcompeting formation of γ-lactams. Overcoming this critical regioselectivity issue, while achieving high enantioselectivity at the same time, remains highly challenging for the synthesis of β-lactams. In addition, the use of costly rare earth metals can pose limitations in many aspects.
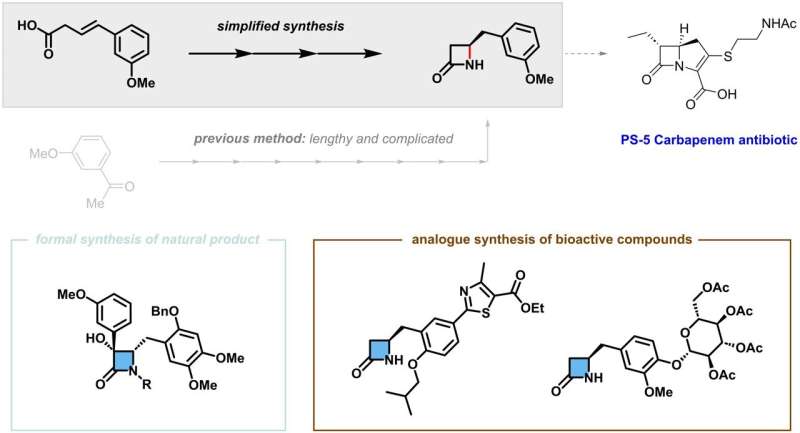
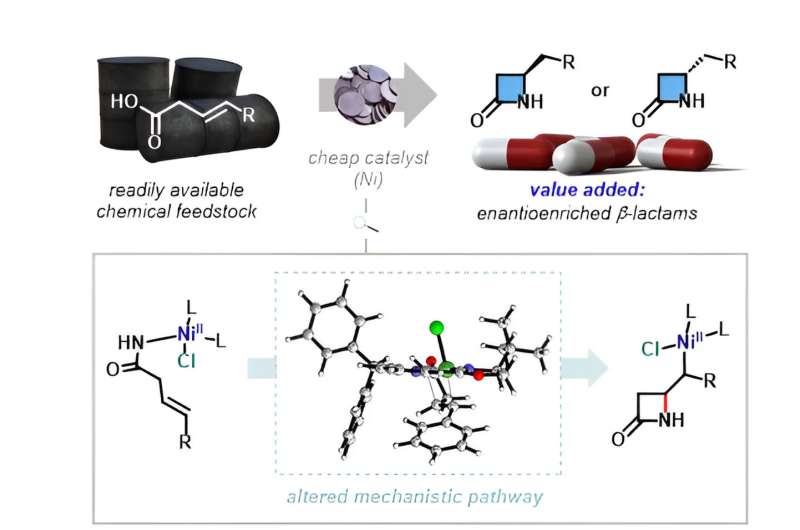
To address these challenges, the IBS research team pioneered a novel catalytic reaction using nickel, which is a far more naturally abundant transition metal. Their work has been published in Nature Catalysis.
They first tackled the challenge in suppressing the formation of five-membered γ-lactams by harnessing the catalytic properties of nickel-hydride (NiH) species and the alkene dioxazolone substrate. High regioselectivity is achieved toward the desired formation of β-lactam scaffolds under this new catalytic system, whereby the NiH species react more favorably with the dioxazolone motif than with alkene to induce a β-selective carbon-nitrogen bond formation via nickel-amido intermediates.
Enantioselective formation of chiral β-lactams has also become possible through combination with chiral ligands, which can be applied to the synthesis of biorelevant compounds.
The researchers at IBS further demonstrated the value of their findings by simplified synthesis of a number of β-lactam compounds that previously required more complicated processes. Moreover, the produced β-lactams are estimated to have high market values up to 700 times in comparison to the starting raw materials, and the fact that this can be achieved using low-cost nickel catalyst makes this process highly economically desirable.
What makes this development stand out is its immediate applicability. By employing this method, the team proposed more efficient and simplified synthesis strategies for specific medicines and natural substances. Moreover, they accomplished the synthesis of new compounds that can be potential drug candidates through late-stage functionalization of complex chemical structures.
Director Chang commented, "Through this study, we were able to easily synthesize chiral β-lactam, the backbone of major antibiotics such as penicillin and carbapenem from cheap nickel catalysts and feedstock chemicals. Discovery of new pathways to synthesize high-value materials like chiral β-lactam is a significant achievement that can greatly shorten the drug discovery phase."
More information: Intramolecular Hydroamidation of Alkenes Enabling Asymmetric Synthesis of β-Lactams via Transposed NiH Catalysis, Nature Catalysis (2023). DOI: 10.1038/s41929-023-01014-2
Journal information: Nature Catalysis
Provided by Institute for Basic Science