New strategy for the synthesis of axially chiral allenic compounds
Recently, a Chinese research team led by Prof. Guo Chang at the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) managed to catalyze an asymmetric propargyl substitution reaction with a nickel-derived catalyst, successfully synthesizing a series of chiral dienes in a diversified route.
Their work was published in Nature Communications and Journal of the American Chemical Society.
Due to their unique structural characteristics, allenic compounds have been widely used in synthetic chemistry, pharmaceutical chemistry and materials science. They also function as important structures in a wide range of efficient construction of various bioactive molecules. The development of efficient and highly selective synthesis methods to construct novel chiral allenic compounds has been a research focus.
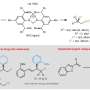
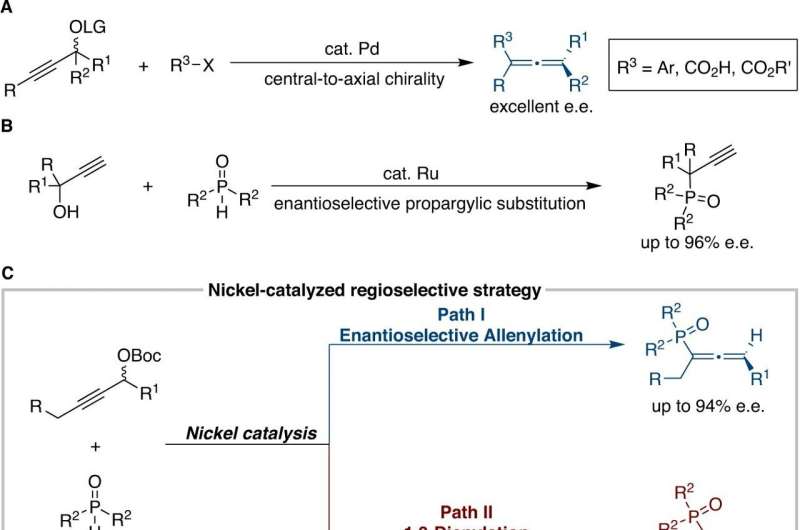
The researchers managed to realize the diversified phosphonylation of racemic propargyl carbonate and synthesized a series of tertiary phosphine oxides effectively by applying two different ligands. One is to synthesize functionalized phosphinoyl 1,3-butadienes with the ligand dcybpz. While the other one is realizing the construction of versatile chiral allenylphosphoryl derivatives with high enantiopurity by applying the newly developed BDPP-type ligand.
Additionally, the team designed a series of N-sulfonyl hydrazide reagents, which were used in the nickel catalyzed asymmetric propargyl substitution/Myers rearrangement tandem reaction, achieving a high stereoselectivity in the synthesis of 1,3-disubstituted allenic compounds. This powerful strategy can be applied in the synthesis of natural allenic compounds, indicating its great potential.
More information: Jiayin Zhang et al, Nickel-catalyzed switchable 1,3-dienylation and enantioselective allenylation of phosphine oxides, Nature Communications (2022). DOI: 10.1038/s41467-022-34764-x
Xianghong Xu et al, Nickel-Catalyzed Asymmetric Propargylation for the Synthesis of Axially Chiral 1,3-Disubstituted Allenes, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c10863
Journal information: Journal of the American Chemical Society , Nature Communications
Provided by Chinese Academy of Sciences