New route to the synthesis of P-chiral compounds
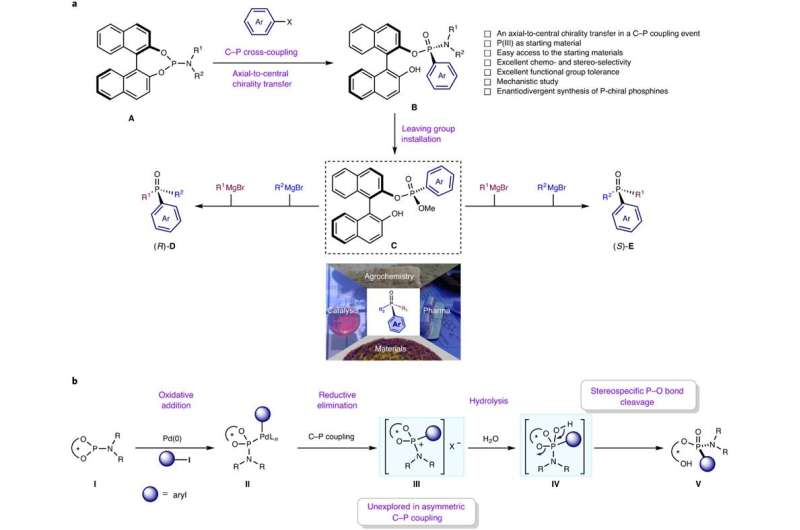
Chiral phosphines are one of the most commonly used ligands in assymetric metal catalysis for the synthesis of various useful drugs and pharmaceuticals. Have you ever wondered how to obtain them? Most of the so-called chiral phosphines are C-stereogenic, which means that the chirality is located at a site other than the P-center. Many of them are currently commercially available. However, the true P-chiral phosphines, in which the chirality is found in the P-center itself, are the most difficult to synthesize in the laboratory because the formation of the C-P bond cannot be controlled. One of the best known methods for C-P bond formation is a metal-catalyzed C-P cross-coupling reactions. However, the control of chirality, i.e. the formation of a single chiral enantiomer, is challenging.
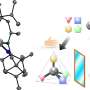
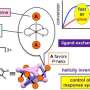
Researchers at the University of Groningen, led by 2016 Nobel Chemistry Prize winner Ben L. Feringa, have solved this problem of assymetric metal catalyzed C-P bond formation by choosing an axially chiral 1,1'–bi–2–naphthol (BINOL)–based phosphoramidite (also known as Feringas ligand) and aryl halides or triflates as starting materials, which was published in Nature Catalysis. The reason for this success is the presence of BINOL, which controls the formation of a single isomer through a process known as axial-central chirality transfer. Although BINOLs are cheap (£38.00/10g in Fluorochem Uk), the researchers have also shown that it can be recycled with high yield and eantiopurity. "The biggest challenge in assymetric cross-coupling to form C-P bonds is the competitive ligand coordination between the external chiral phosphine ligand and the P-containing substrate/product. The key to our success is that the BINOL-containing phosphoramidites have the properties of an intrinsic chiral ligand and can serve as a substrate at the same time," adds one of the first authors, Anirban Mondal, a Ph.D. student in Feringa's group.
Moreover, the unique finding of this work is the formation of an unexplored P-chiral phosphonium salt intermediate, genreted after arylation step, that can be visualized by X-ray crystallography. According to the authors, this unique phosphonium salt, in which the chirality is located in the P-center, can open a new field as a chiral phase transfer catalyst or serve as a starting material for many other P-chiral compounds.
"When we first developed phosphoramidites as ligands in 1996, we were intrigued by their excellent stereocontrol in copper-catalyzed C-C bond formation, which led to a breakthrough in catalytic asymmetric conjugate addition. As phosphoramidites found use in industry, we imagined utilizing them given their unique chiral properties,as starting reagents for asymmetric transformations. Traditionally, an external chiral ligand is used for chiral induction in a C-P coupling reaction, but the competitive coordination of initial and final phosphorus compounds with the metal catalysts, together with an external chiral ligand, reduces the enantioselectivity. As BINOL-containing phosphoramidites have the properties of an intrinsic chiral ligand and simultaneously can serve as a substrate, we hypothesized that they would increase stereoselectivity in C-P coupling processes with aryl compounds, and were delighted when our data confirmed this," adds Dr. Feringa in a research briefing published in Nature Catalysis.
The broad applicability and high flexibility of the phosphorus derivatives and synthetic steps, together with an intelligent approach to chirality transfer through the cheap and readily available BINOL method, open up a new route by which many difficult-to-access P-chirogenic compounds can be synthesized for many purposes, including drug discovery, materials chemistry, organoneucliotide chemistry, and especially as ligands for metal catalysis.
Mondal adds, "With this current method, we can now synthesize various P-chiral phoshine ligands with both mirror image forms, opening up a new field of research. Ultimately, this will allow us to develop methods in which P-chirality plays an important role."
More information: Anirban Mondal et al, P-chirogenic phosphorus compounds by stereoselective Pd-catalysed arylation of phosphoramidites, Nature Catalysis (2021). DOI: 10.1038/s41929-021-00697-9
Journal information: Nature Catalysis
Provided by University of Groningen