This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Successful development of Pauson–Khand reaction with atropisomeric substrates could result in new practical applications
The rich diversity of organic compounds is a result of the remarkable ability of carbon atoms to connect and form bonds with different molecules. Variations in these bonding arrangements, as well as the types of atoms and functional groups involved, result in the formation of isomers. They are a type of compound that shares the same molecular formula but exhibits distinct three-dimensional shapes and properties.
Atropisomers are a type of isomers that are formed when bulky substituents or functional groups become attached to a single bond and experience restricted rotation about the bond. This restriction leads to molecules with a distinct spatial arrangement of atoms and functional groups. Atropisomers are often encountered in compounds containing aromatic rings.
Notably, compounds formed as a result of restricted rotation around an N−C single bond between the aromatic ring and the functional group are used as chiral ligands and chiral building blocks to create specific stereoisomers with chiral carbon centers. They often find use in various chemical and pharmaceutical applications. However, maintaining axial chirality around the N−C bond is challenging to attain, and this results in a limited number of synthesizable N−C axially chiral compounds.
In a recent study, researchers have now demonstrated that when the Pauson–Khand reaction, a chemical reaction for synthesizing molecules containing cyclopentenones, is applied to atropisomeric substrates, it can produce new derivatives containing a chiral carbon. The study was published in the journal Organic Letters.
The research was led by Professor Osamu Kitagawa from the Department of Applied Chemistry at Shibaura Institute of Technology, Japan, along with graduate students Ryohei Kasahara and Tatsuya Toyoda. Possibly the first demonstration of this kind, this breakthrough significantly expands the scope of synthesizing such compounds.
"Although [the] Pauson–Khand reaction is a widely used reaction for the synthesis of many organic compounds, the reaction with atropisomeric substrates had not been explored yet. Out of academic curiosity, we decided to explore the Pauson–Khand reaction with atropisomeric substrates," says Prof. Kitagawa, while talking about the study.
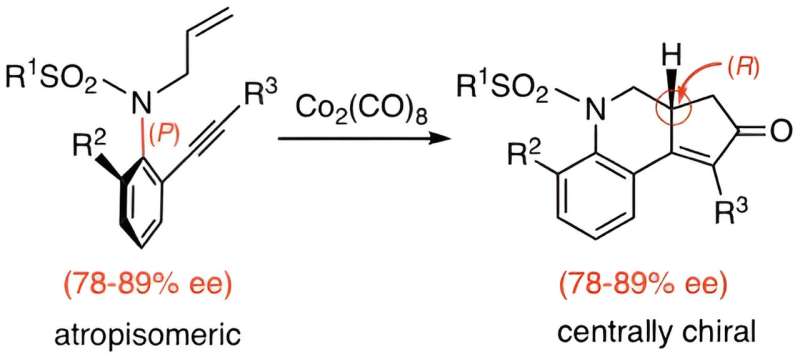
To this end, the researchers applied an intramolecular version of the reaction to enantioenriched atropisomeric sulfonamides for synthesizing chiral nitrogen-containing tricyclic compounds. They observed that conducting Pauson–Khand reactions on enantioenriched atropisomeric sulfonamides (78–89% ee) containing various substituents in the presence of a Co2(CO)8 complex led to the formation of products with enantiomeric excesses ranging from 78% to 89%.
These findings suggest that the Pauson–Khand reaction can effectively transfer chirality from the starting materials to the reaction products with a high degree of selectivity.
"The reaction with enantioenriched N–C axially chiral sulfonamide derivatives proceeded with complete chirality transfer from axial chirality (P configuration) to central chirality (R configuration), affording chiral nitrogen-containing tricyclic compounds," explains Prof. Kitagawa.
Further, a wide range of substrates were compatible with the reaction, including sulfonamides with methyl-, chloro-, and bromo- substituents, as well as aromatic groups. A crucial factor for the success of this approach was the presence of the Co2(CO)8 complex, which forms an intermediate with the substrate. The specific arrangement of carbon and cobalt atoms within this intermediate controls how alkenes attach to it, promoting the formation of specific enantiomers.
In summary, this level of control over chirality is of significant importance in chemical synthesis and has practical applications in various fields, particularly for the synthesis of pharmaceutical compounds.
"With the reaction products possessing a nitrogen-containing tricyclic structure, the reaction can be used for novel applications, such as for the synthesis of natural products and bioactive compounds," concludes Prof. Kitagawa.
More information: Ryohei Kasahara et al, Chirality Transfer Intramolecular Pauson–Khand Reaction with N–C Axially Chiral Sulfonamides Bearing an Ene–Yne Structure, Organic Letters (2023). DOI: 10.1021/acs.orglett.3c02893
Journal information: Organic Letters
Provided by Shibaura Institute of Technology