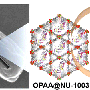
A new fabric coating could neutralize chemical weapons and help save countless lives. Credit: American Chemical Society
Chemical weapons are nightmarish. In a millisecond, they can kill hundreds, if not thousands. But, in a study published in the ACS journal Chemistry of Materials, scientists report that they have developed a way to adhere a lightweight coating onto fabrics that is capable of neutralizing a subclass of these toxins—those that are delivered through the skin. The life-saving technique could eventually be used to protect soldiers and emergency responders.
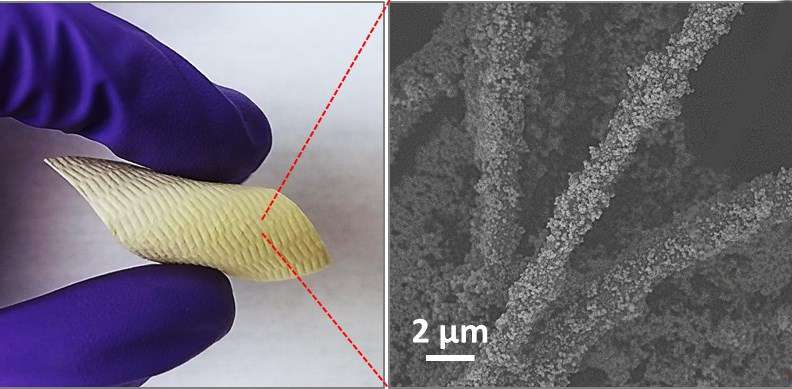
Since their first use in World War I, dozens of chemical weapons with devastating potential have been developed. For example, just a pinprick-sized droplet of the nerve gas sarin on the skin is lethal. Recently, scientists have begun exploring the use of zirconium-based metal-organic framework (MOF) powders to degrade and destroy these harmful compounds. MOFs are miniscule, porous structures that have large surface areas that allow them to absorb vast amounts of gases and other substances. The zirconium within them helps neutralize toxic materials. But making MOFs can be tedious, requiring high temperatures and long reaction times. Plus, most MOF powders are unstable and incorporating them onto clothing has proven challenging. Dennis Lee, Gregory N. Parsons and colleagues wanted to see if they could "grow" MOFs onto fabric at room temperature, potentially creating a lightweight shield that could be used on uniforms and protective clothing.
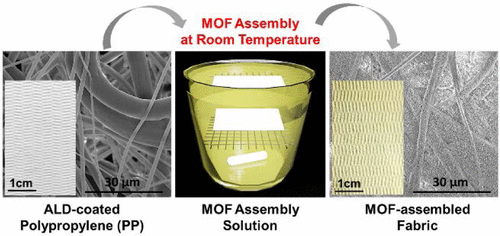
Building on previous work, the researchers exposed polypropylene, a nonwoven fabric commonly used in reusable shopping bags and some clothing, to a mixture consisting of a zirconium-based MOF, a solvent and two binding agents. To ensure that the coating spread evenly across the cloth, they treated the fabrics with thin layers of aluminum, titanium or zinc oxide. They tested this combination with dimethyl 4-nitrophenyl phosphate (DMNP), a relatively harmless molecule that has similar reactivity as sarin, soman and other nerve agents. They found that the MOF-treated cloths deactivated the DMNP in less than 5 minutes, suggesting this process is a viable means to create improved protective clothing.
Credit: American Chemical Society
More information: Dennis T. Lee et al. Catalytic "MOF-Cloth" Formed via Directed Supramolecular Assembly of UiO-66-NHCrystals on Atomic Layer Deposition-Coated Textiles for Rapid Degradation of Chemical Warfare Agent Simulants, Chemistry of Materials (2017). DOI: 10.1021/acs.chemmater.7b00949
Abstract
Highly tunable metal–organic framework (MOF) materials, including, for example, UiO-66-NH2, are known to be effective catalysts to degrade chemical warfare agents (CWAs) with half-lives near 1 min. Therefore, many researchers have been actively working on producing supported MOF materials to improve application effectiveness by using relatively slow solvothermal synthesis or repetitious stepwise layer-by-layer methods. Herein, we demonstrate a facile route to rapidly assemble presynthesized UiO-66-NH2 crystals onto nonwoven polypropylene (PP) fibrous mats at ambient temperature. Crystal assembly is chemically directed using β-cyclodextrin (β-CD) and cetyltrimethylammonium bromide (CTAB) as surfactant assembly agents, where the agents quickly (within 5 min) self-assemble on the crystal surface and promote physically robust chemical surface attachment while simultaneously impeding solution-phase crystal agglomeration. Furthermore, we find that when the PP is preconditioned using conformal metal oxide thin films, including Al2O3, TiO2, or ZnO formed via atomic layer deposition (ALD), the hydrophilic metal oxide surface further helps improve assembly uniformity and MOF mass loading, producing MOF crystal loading as high as 40 wt % and an overall BET surface area exceeding 200 m2/g(MOF+Fiber). Using these surface-assembled MOFs, we observe catalytic degradation of dimethyl 4-nitrophenyl phosphate (DMNP), a CWA simulant, with a half-life of less than 5 min.
Journal information: Chemistry of Materials
Provided by American Chemical Society