(Phys.org)—Self-assembled monolayers are organic molecules that spontaneously coordinate to a metal surface. If this metal surface is an electrode, then a current can pass through the organic monolayer and interact with a second electrode on the terminal side of the molecule. This current can be controlled by changing the characteristics of the organic molecule, such as making it longer or adding polar substituents or other functional groups. This tailoring of organic circuits is part of a bigger project of creating organic-based electronics.
One way to quantitatively compare one monolayer to another is to measure electron tunneling between the electrode and the junction point at the terminal end of the monolayer. Lead author, Kung-Ching Liao of George M. Whitesides's group at Harvard University investigated whether decreasing the strength of the interaction between the monolayer's terminal group and the Ga2O3 interface would decrease the tunneling current density of the monolayer. Their work was published in the Journal of the American Chemical Society.
Prior studies by Whitesides's group looked at how electron tunneling changes when the atom that is coordinated to the metal electrode (the anchor) is changed, or when the organic components (the backbone) between the anchor and the terminal group are changed. Their prior research revealed that increasing the strength of the terminal group interaction with Ga2O3 did not increase tunneling current density; however, decreasing the interaction between the terminal group and Ga2O3 junction seemed to decrease it. In this study, Liao et al. sought to investigate the mechanism for this decrease in tunneling current density.
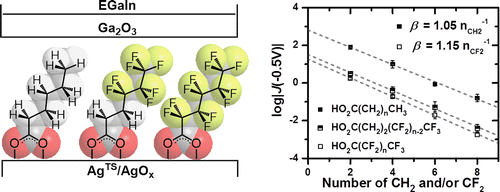
To understand this mechanism, they needed to make a direct comparison between two similar monolayers and their terminal groups. They chose to compare a monolayer comprised of C-H bonds with a monolayer that has some or all C-H bonds replaced with C-F bonds. The C-F bond is less polarizable than the C-H bond and has a longer bond length than C-H. Additionally, changing the hydrogens on an aliphatic carbon chain to fluorine decreases the energy of the HOMO and, in a monolayer, disrupts van der Waals interactions between alkyl chains. By investigating the effects of changing these factors, Liao et al. were able to discern the primary cause for the change in electron tunneling density.
They found that changing the backbone from (CH2)n to (CF2)n had a small, but noticeable effect on the tunneling density. This effect was not enough to account for the differences noted in prior experiments. However, when they changed the terminal group from CH3 to CF3, they found that this factor alone, accounted for the change in electron tunneling density.
Whether the backbone was an n-alkyl chain or para-substituted omega-tolyl-alkanoates or oligophenyl carboxylates, the rate of charge transport across the CF3 terminal group and Ga2O3 from the EGaIn tip differed from the CH3 equivalent by a factor of 25 to 30.
This discovery allows for additional fine-tuning of an organic circuit by selecting a particular backbone and anchor with desirable electronic properties. It also sheds light on the importance of the terminal group junction interaction.
More information: "Fluorination and tunneling across molecular junctions" J. Am. Chem. Soc., 2015, 137 (11), pp 3852–3858. DOI: 10.1021/jacs.5b00137
Abstract
This paper describes the influence of the substitution of fluorine for hydrogen on the rate of charge transport by hole tunneling through junctions of the form AgTSO2C(CH2)n(CF2)mT//Ga2O3/EGaIn, where T is methyl (CH3) or trifluoromethyl (CF3). Alkanoate-based self-assembled monolayers (SAMs) having perfluorinated groups (RF) show current densities that are lower (by factors of 20–30) than those of the homologous hydrocarbons (RH), while the attenuation factors of the simplified Simmons equation for methylene (β = (1.05 ± 0.02)nCH2–1) and difluoromethylene (β = (1.15 ± 0.02)nCF2–1) are similar (although the value for (CF2)n is statistically significantly larger). A comparative study focusing on the terminal fluorine substituents in SAMs of ω-tolyl- and -phenyl-alkanoates suggests that the C–F//Ga2O3 interface is responsible for the lower tunneling currents for CF3. The decrease in the rate of charge transport in SAMs with RF groups (relative to homologous RH groups) is plausibly due to an increase in the height of the tunneling barrier at the T//Ga2O3 interface, and/or to weak van der Waals interactions at that interface.
Journal information: Journal of the American Chemical Society
© 2015 Phys.org