Credit: Nano-Micro Letters (2024). DOI: 10.1007/s40820-024-01375-8
With the depletion of fossil fuels and global warming, there is an urgent need to seek green, clean, and efficient energy resources. Against this backdrop, hydrogen is considered a potential candidate for replacing fossil fuels due to its high energy density and environmentally friendly nature. To realize the development of a hydrogen economy, safe and efficient hydrogen storage technologies are crucial.
Compared to traditional compressed hydrogen and cryogenic liquid hydrogen storage technologies, solid-state hydrogen storage is considered a safer and more efficient method. Magnesium hydride (MgH2), as one of the most promising solid-state hydrogen storage materials, has attracted attention due to its abundant elemental resources, high hydrogen storage capacity, good reversibility, and non-toxicity. However, the relatively high operating temperature of MgH2 limits its large-scale commercial application in vehicular or stationary hydrogen storage.
Introducing transition metal-based catalysts with unique three-dimensional electronic structures is considered an effective method to improve the kinetics of MgH2. Vanadium (V) and its oxides are often used as catalysts for MgH2 due to their multivalence and high catalytic activity. However, due to the high ductility of metallic vanadium and relatively low activity, vanadium-based oxides have broader application prospects.
Layered V2O5 with a layered structure is one of the promising catalysts to enhance the hydrogen storage performance of MgH2/Mg, but limited catalytic capacity due to insufficient contact between V2O5 and MgH2.
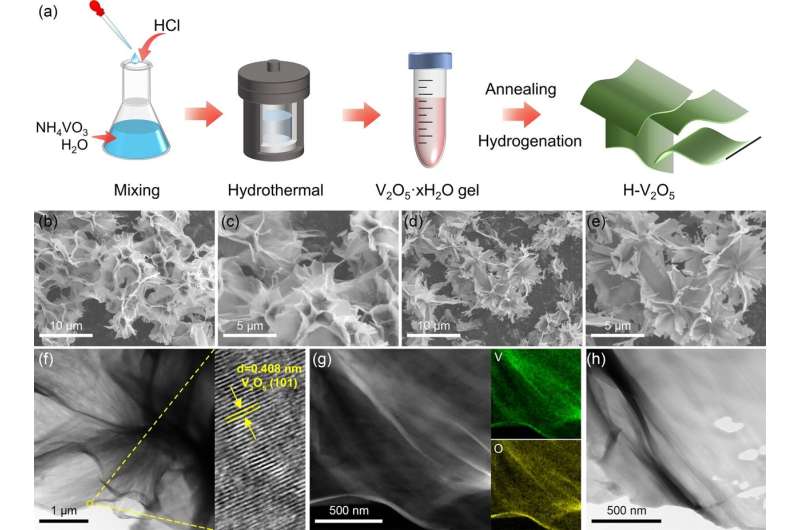
To address this issue, Dr. Jianxin Zou's team at Shanghai Jiao Tong University employed a solvothermal method followed by subsequent hydrogenation to prepare ultra-thin hydrogenated V2O5 nanosheets with abundant oxygen vacancies and used them as catalysts to improve the hydrogen storage performance of MgH2.
The study is published in the journal Nano-Micro Letters.
The MgH2-H-V2O5 composite material exhibits excellent hydrogen storage performance, including a lower desorption temperature (Tonset = 185°C), rapid desorption kinetics (Ea = 84.55 kJ mol−1 H2 for desorption), and long-term cyclic stability (capacity retention of up to 99% after 100 cycles). Particularly, the MgH2-H-V2O5 composite material shows outstanding hydrogen absorption performance at room temperature, with a hydrogen absorption capacity of 2.38 wt% within 60 minutes at 30°C.
The H-V2O5 nanosheets synthesized by Dr. Zou's team possess a unique two-dimensional structure and abundant oxygen vacancies, enabling the in-situ formation of V/VH2 during the reaction process, all of which contribute to enhancing the hydrogen storage performance of MgH2.
By using a solvothermal method to create a distinct anisotropic layered structure, a highly exposed surface is formed, thereby providing more active sites and pathways for hydrogen/electron diffusion, thus improving hydrogen storage performance. Moreover, crucially, the presence of oxygen vacancies accelerates electron transfer, stimulating the "hydrogen pump" effect of VH2/V, facilitating the dehydrogenation of VH2 and MgH2, and reducing the energy barriers for hydrogen dissociation and recombination.
Introducing oxygen vacancy defect engineering into the catalyst thus opens up a new avenue for enhancing the cyclic stability and kinetic performance of MgH2.
More information: Li Ren et al, Boosting Hydrogen Storage Performance of MgH2 by Oxygen Vacancy-Rich H-V2O5 Nanosheet as an Excited H-Pump, Nano-Micro Letters (2024). DOI: 10.1007/s40820-024-01375-8
Provided by Shanghai Jiao Tong University Journal Center